Docosahexaenoic (DHA) modulates phospholipid-hydroperoxide glutathione peroxidase (Gpx4) gene expression to ensure self-protection from oxidative damage in hippocampal cells
- PMID: 26257655
- PMCID: PMC4510835
- DOI: 10.3389/fphys.2015.00203
Docosahexaenoic (DHA) modulates phospholipid-hydroperoxide glutathione peroxidase (Gpx4) gene expression to ensure self-protection from oxidative damage in hippocampal cells
Abstract
Docosahexaenoic acid (DHA, 22:6n-3) is a unique polyunsaturated fatty acid particularly abundant in nerve cell membrane phospholipids. DHA is a pleiotropic molecule that, not only modulates the physicochemical properties and architecture of neuronal plasma membrane, but it is also involved in multiple facets of neuronal biology, from regulation of synaptic function to neuroprotection and modulation of gene expression. As a highly unsaturated fatty acid due to the presence of six double bonds, DHA is susceptible for oxidation, especially in the highly pro-oxidant environment of brain parenchyma. We have recently reported the ability of DHA to regulate the transcriptional program controlling neuronal antioxidant defenses in a hippocampal cell line, especially the glutathione/glutaredoxin system. Within this antioxidant system, DHA was particularly efficient in triggering the upregulation of Gpx4 gene, which encodes for the nuclear, cytosolic, and mitochondrial isoforms of phospholipid-hydroperoxide glutathione peroxidase (PH-GPx/GPx4), the main enzyme protecting cell membranes against lipid peroxidation and capable to reduce oxidized phospholipids in situ. We show here that this novel property of DHA is also significant in the hippocampus of wild-type mice and, to a lesser extent in APP/PS1 transgenic mice, a familial model of Alzheimer's disease. By doing this, DHA stimulates a mechanism to self-protect from oxidative damage even in the neuronal scenario of high aerobic metabolism and in the presence of elevated levels of transition metals, which inevitably favor the generation of reactive oxygen species. Noticeably, DHA also upregulated a Gpx4 CIRT (Cytoplasmic Intron-sequence Retaining Transcripts), a novel Gpx4 splicing variant, harboring part of the first intronic region, which according to the "sentinel RNA hypothesis" would expand the ability of Gpx4 (and DHA) to provide neuronal antioxidant defense independently of conventional nuclear splicing in cellular compartments, like dendritic zones, located away from nuclear compartment. We discuss here, the crucial role of this novel transcriptional regulation triggered by DHA in the context of normal and pathological hippocampal cell.
Keywords: docosahexaenoic acid; glutathione peroxidase 4; hippocampal cells; intron retention; neuroprotection; phospholipid-hydroperoxide glutathione peroxidase; transcriptional regulation.
Figures
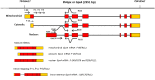
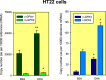


References
-
- Aso E., Lomoio S., López-Gonzalez I., Joda L., Carmona M., Fernández-Yagüe N., et al. . (2012). Amyloid generation and dysfunctional immunoproteasome activation with disease progression in animal model of familial Alzheimer disease. Brain Pathol. 22, 636–653. 10.1111/j.1750-3639.2011.00560.x - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

