Modulating the Innate Immune Response to Influenza A Virus: Potential Therapeutic Use of Anti-Inflammatory Drugs
- PMID: 26257731
- PMCID: PMC4507467
- DOI: 10.3389/fimmu.2015.00361
Modulating the Innate Immune Response to Influenza A Virus: Potential Therapeutic Use of Anti-Inflammatory Drugs
Abstract
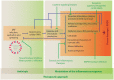
Infection by influenza A viruses (IAV) is frequently characterized by robust inflammation that is usually more pronounced in the case of avian influenza. It is becoming clearer that the morbidity and pathogenesis caused by IAV are consequences of this inflammatory response, with several components of the innate immune system acting as the main players. It has been postulated that using a therapeutic approach to limit the innate immune response in combination with antiviral drugs has the potential to diminish symptoms and tissue damage caused by IAV infection. Indeed, some anti-inflammatory agents have been shown to be effective in animal models in reducing IAV pathology as a proof of principle. The main challenge in developing such therapies is to selectively modulate signaling pathways that contribute to lung injury while maintaining the ability of the host cells to mount an antiviral response to control virus replication. However, the dissection of those pathways is very complex given the numerous components regulated by the same factors (i.e., NF kappa B transcription factors) and the large number of players involved in this regulation, some of which may be undescribed or unknown. This article provides a comprehensive review of the current knowledge regarding the innate immune responses associated with tissue damage by IAV infection, the understanding of which is essential for the development of effective immunomodulatory drugs. Furthermore, we summarize the recent advances on the development and evaluation of such drugs as well as the lessons learned from those studies.
Keywords: ARDS; anti-inflammatory therapy; cytokines; inflammation; influenza virus; innate immunity.
Figures
References
-
- CDC. People at High Risk of Developing Flu-Related Complications (2015). Available from: http://www.cdc.gov/flu/about/disease/high_risk.htm
-
- CDC. 2014-2015 Influenza season week 10 ending March 14, 2015. Fluview. Influenza Hospitalization Surveillance Network (FluSurv-NET) (2015). Available from: http://www.cdc.gov/flu/weekly/pdf/External_F1516.pdf
-
- CDC. Estimates of deaths associated with seasonal influenza – United States, 1976-2007. MMWR Morb Mortal Wkly Rep (2010) 59:1057–62. - PubMed
-
- CDC. Interim Risk Assessment and Biosafety Level Recommendations for Working With Influenza A(H7N9) Viruses (2013). Available from: http://www.cdc.gov/flu/avianflu/h7n9/risk-assessment.htm
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

