Purification and Structural Characterization of "Simple Catechol", the NGAL-Siderocalin Siderophore in Human Urine
- PMID: 26257890
- PMCID: PMC4527557
- DOI: 10.1039/C5RA02509E
Purification and Structural Characterization of "Simple Catechol", the NGAL-Siderocalin Siderophore in Human Urine
Abstract
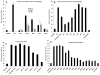
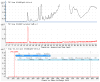
The identification of ligands that bind the protein Neutrophil Gelatinase-Associated Lipocalin (NGAL, Siderocalin, Lipocalin-2) have helped to elucidate its function. NGAL-Siderocalin binds and sequesters the iron loaded bacterial siderophore enterochelin (Ent), defining the protein as an innate immune effector. Simple metabolic catechols can also form tight complexes with NGAL-Siderocalin and ferric iron, suggesting that the protein may act as an iron scavenger even in the absence of Ent. While different catechols have been detected in human urine, they have not been directly purified from a biofluid and demonstrated to ligate iron with NGAL-Siderocalin. This paper describes a "natural products" approach to identify small molecules that mediate iron binding to NGAL-Siderocalin. A 10K filtrate of human urine was subjected to multiple steps of column chromatography and reverse-phase HPLC, guided by NGAL-Siderocalin-iron binding assays and LC-MS detection. The co-factor forming a ternary structure with iron and NGAL-Siderocalin was identified as authentic simple catechol (dihydroxybenze) by ESI-HR-Mass, UV, and NMR spectrometric analysis. Comparison of the binding strengths of different catechols demonstrated that the vicinal-dihydroxyl groups were the key functional groups and that steric compatibilities of the catechol ring have the strongest effect on binding. Although catechol was a known NGAL-Siderocalin co-factor, our purification directly confirmed its presence in urine as well as its capacity to serve as an iron trap with NGAL-Siderocalin.
Keywords: Binding; Catechol; Enterochelin; Ferric Iron; Lipocalin-2; NGAL; Purification; Siderocalin.
Figures



References
-
- Triebel S, Blaser J, Reinke H, Tschesche H. A 25kDa alpha 2-microglobulin related protein is a component of the 125 kDa form of human gelatinase. FEBS Lett. 1992;314:386–388. - PubMed
-
- Kjeldsen L, Johnsen AH, Sengelov H, Borregaard N. Isolation and primary structure of NGAL, a novel protein associated with human neutrophil gelatinase. J Biol Chem. 1993;268:10425–10432. - PubMed
-
- Goetz DH, Holmes MA, Borregaard N, Bluhm ME, Raymond KN, et al. The neutrophil Lipocalin NGAL is a bacteriostatic agent that interferes with siderophore-mediated iron acquisition. Mol Cell. 2002;10:1033–1043. - PubMed
-
- Flo TH, Smith KD, Sato S, Rodriguez DJ, Holmes MA, et al. Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature. 2004;432:917–921. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
