Innate Immune Defenses in Human Tuberculosis: An Overview of the Interactions between Mycobacterium tuberculosis and Innate Immune Cells
- PMID: 26258152
- PMCID: PMC4516846
- DOI: 10.1155/2015/747543
Innate Immune Defenses in Human Tuberculosis: An Overview of the Interactions between Mycobacterium tuberculosis and Innate Immune Cells
Abstract
Tuberculosis (TB) remains a serious global public health problem that results in up to 2 million deaths each year. TB is caused by the human pathogen, Mycobacterium tuberculosis (Mtb), which infects primarily innate immune cells patrolling the lung. Innate immune cells serve as barometers of the immune response against Mtb infection by determining the inflammatory milieu in the lungs and promoting the generation of adaptive immune responses. However, innate immune cells are also potential niches for bacterial replication and are readily manipulated by Mtb. Our understanding of the early interactions between Mtb and innate immune cells is limited, especially in the context of human infection. This review will focus on Mtb interactions with human macrophages, dendritic cells, neutrophils, and NK cells and detail evidence that Mtb modulation of these cells negatively impacts Mtb-specific immune responses. Furthermore, this review will emphasize important innate immune pathways uncovered through human immunogenetic studies. Insights into the human innate immune response to Mtb infection are necessary for providing a rational basis for the augmentation of immune responses against Mtb infection, especially with respect to the generation of effective anti-TB immunotherapeutics and vaccines.
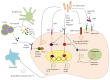
Figures



References
-
- World Health Organization. Global Tuberculosis Report 2014. WHO; 2014.
-
- Dye C., Scheele S., Dolin P., Pathania V., Raviglione M. C. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. WHO Global Surveillance and Monitoring Project. Journal of the American Medical Association. 1999;282(7):677–686. doi: 10.1001/jama.282.7.677. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

