Rapamycin improves TIE2-mutated venous malformation in murine model and human subjects
- PMID: 26258417
- PMCID: PMC4588237
- DOI: 10.1172/JCI76004
Rapamycin improves TIE2-mutated venous malformation in murine model and human subjects
Abstract
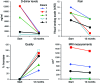
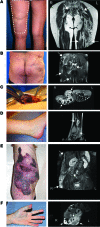
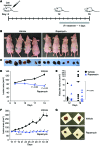
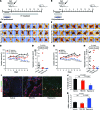
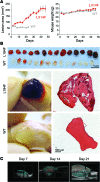
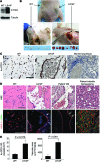
Venous malformations (VMs) are composed of ectatic veins with scarce smooth muscle cell coverage. Activating mutations in the endothelial cell tyrosine kinase receptor TIE2 are a common cause of these lesions. VMs cause deformity, pain, and local intravascular coagulopathy, and they expand with time. Targeted pharmacological therapies are not available for this condition. Here, we generated a model of VMs by injecting HUVECs expressing the most frequent VM-causing TIE2 mutation, TIE2-L914F, into immune-deficient mice. TIE2-L914F-expressing HUVECs formed VMs with ectatic blood-filled channels that enlarged over time. We tested both rapamycin and a TIE2 tyrosine kinase inhibitor (TIE2-TKI) for their effects on murine VM expansion and for their ability to inhibit mutant TIE2 signaling. Rapamycin prevented VM growth, while TIE2-TKI had no effect. In cultured TIE2-L914F-expressing HUVECs, rapamycin effectively reduced mutant TIE2-induced AKT signaling and, though TIE2-TKI did target the WT receptor, it only weakly suppressed mutant-induced AKT signaling. In a prospective clinical pilot study, we analyzed the effects of rapamycin in 6 patients with difficult-to-treat venous anomalies. Rapamycin reduced pain, bleeding, lesion size, functional and esthetic impairment, and intravascular coagulopathy. This study provides a VM model that allows evaluation of potential therapeutic strategies and demonstrates that rapamycin provides clinical improvement in patients with venous malformation.
Figures


References
-
- Boon LM, Mulliken JB, Enjolras O, Vikkula M. Glomuvenous malformation (glomangioma) and venous malformation: distinct clinicopathologic and genetic entities. Arch Dermatol. 2004;140(8):971–976. - PubMed
-
- Brouillard P, Vikkula M. Genetic causes of vascular malformations. Hum Mol Genet. 2007;16 Spec No. 2:R140–R149. - PubMed
-
- Burrows PE, Mason KP. Percutaneous treatment of low flow vascular malformations. J Vasc Interv Radiol. 2004;15(5):431–445. doi: 10.1097/01.RVI.0000124949.24134.CF. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

