A Non-Inferiority, Individually Randomized Trial of Intermittent Screening and Treatment versus Intermittent Preventive Treatment in the Control of Malaria in Pregnancy
- PMID: 26258474
- PMCID: PMC4530893
- DOI: 10.1371/journal.pone.0132247
A Non-Inferiority, Individually Randomized Trial of Intermittent Screening and Treatment versus Intermittent Preventive Treatment in the Control of Malaria in Pregnancy
Abstract
Background: The efficacy of intermittent preventive treatment for malaria with sulfadoxine-pyrimethamine (IPTp-SP) in pregnancy is threatened in parts of Africa by the emergence and spread of resistance to SP. Intermittent screening with a rapid diagnostic test (RDT) and treatment of positive women (ISTp) is an alternative approach.
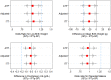
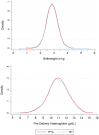
Methods and findings: An open, individually randomized, non-inferiority trial of IPTp-SP versus ISTp was conducted in 5,354 primi- or secundigravidae in four West African countries with a low prevalence of resistance to SP (The Gambia, Mali, Burkina Faso and Ghana). Women in the IPTp-SP group received SP on two or three occasions whilst women in the ISTp group were screened two or three times with a RDT and treated if positive for malaria with artemether-lumefantrine (AL). ISTp-AL was non-inferior to IPTp-SP in preventing low birth weight (LBW), anemia and placental malaria, the primary trial endpoints. The prevalence of LBW was 15.1% and 15.6% in the IPTp-SP and ISTp-AL groups respectively (OR = 1.03 [95% CI: 0.88, 1.22]). The mean hemoglobin concentration at the last clinic attendance before delivery was 10.97g/dL and 10.94g/dL in the IPTp-SP and ISTp-AL groups respectively (mean difference: -0.03 g/dL [95% CI: -0.13, +0.06]). Active malaria infection of the placenta was found in 24.5% and in 24.2% of women in the IPTp-SP and ISTp-AL groups respectively (OR = 0.95 [95% CI 0.81, 1.12]). More women in the ISTp-AL than in the IPTp-SP group presented with malaria parasitemia between routine antenatal clinics (310 vs 182 episodes, rate difference: 49.4 per 1,000 pregnancies [95% CI 30.5, 68.3], but the number of hospital admissions for malaria was similar in the two groups.
Conclusions: Despite low levels of resistance to SP in the study areas, ISTp-AL performed as well as IPTp-SP. In the absence of an effective alternative medication to SP for IPTp, ISTp-AL is a potential alternative to IPTp in areas where SP resistance is high. It may also have a role in areas where malaria transmission is low and for the prevention of malaria in HIV positive women receiving cotrimoxazole prophylaxis in whom SP is contraindicated.
Trial registration: ClinicalTrials.gov NCT01084213 Pan African Clinical trials Registry PACT201202000272122.
Conflict of interest statement
Figures

References
-
- World Health Organisation. Updated WHO policy recommendation: intermittent preventive treatment of malaria in pregnancy using sulfadoxine-pyrimethamine (IPTp-SP). Geneva: World Health Organisation; 2012.
-
- ter Kuile FO, van Eijk AM, Filler SJ. Effect of sulfadoxine-pyrimethamine resistance on the efficacy of intermittent preventive therapy for malaria control during pregnancy: a systematic review. JAMA 2007;297: 2603–2616. - PubMed
-
- Kayentao K, Garner P, van Eijk AM, Naidoo I, Roper C, Mulokozi A, et al. Intermittent preventive therapy for malaria during pregnancy using 2 vs 3 or more doses of sulfadoxine-pyrimethamine and risk of low birth weight in Africa: systematic review and meta-analysis. JAMA 2013;309: 594–604. 10.1001/jama.2012.216231 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

