Oral fumaric acid esters for psoriasis
- PMID: 26258748
- PMCID: PMC6464505
- DOI: 10.1002/14651858.CD010497.pub2
Oral fumaric acid esters for psoriasis
Abstract
Background: Psoriasis is a chronic inflammatory skin condition that can markedly reduce life quality. Several systemic therapies exist for moderate to severe psoriasis, including oral fumaric acid esters (FAE). These contain dimethyl fumarate (DMF), the main active ingredient, and monoethyl fumarate. FAE are licensed for psoriasis in Germany but used off-licence in many countries.
Objectives: To assess the effects and safety of oral fumaric acid esters for psoriasis.
Search methods: We searched the following databases up to 7 May 2015: the Cochrane Skin Group Specialised Register, CENTRAL in the Cochrane Library (Issue 4, 2015), MEDLINE (from 1946), EMBASE (from 1974), and LILACS (from 1982). We searched five trials registers and checked the reference lists of included and excluded studies for further references to relevant randomised controlled trials. We handsearched six conference proceedings that were not already included in the Cochrane Skin Group Specialised Register.
Selection criteria: Randomised controlled trials (RCTs) of FAE, including DMF monotherapy, in individuals of any age and sex with a clinical diagnosis of psoriasis.
Data collection and analysis: Two review authors independently assessed trial quality and extracted data. Primary outcomes were improvement in Psoriasis Area and Severity Index (PASI) score and the proportion of participants discontinuing treatment due to adverse effects.
Main results: We included 6 studies (2 full reports, 2 abstracts, 1 brief communication, and 1 letter), with a total of 544 participants. Risk of bias was unclear in several studies because of insufficient reporting. Five studies compared FAE with placebo, and one study compared FAE with methotrexate. All studies reported data at 12 to 16 weeks, and we identified no longer-term studies. When FAE were compared with placebo, we could not perform meta-analysis for the primary outcome of PASI score because the three studies that assessed this outcome reported the data differently, although all studies reported a significant reduction in PASI scores with FAE. Only 1 small study designed for psoriatic arthritis reported on the other primary outcome of participants discontinuing treatment due to adverse effects (2 of 13 participants on FAE compared with none of the 14 participants on placebo; risk ratio (RR) 5.36, 95% confidence interval (CI) 0.28 to 102.1; 27 participants; very low-quality evidence). However, these findings are uncertain due to indirectness and a very wide confidence interval. Two studies, containing 247 participants and both only reported as abstracts, allowed meta-analysis for PASI 50, which showed superiority of FAE over placebo (RR 4.55, 95% CI 2.80 to 7.40; low-quality evidence), with a combined PASI 50 of 64% in those given FAE compared with a PASI 50 of 14% for those on placebo, representing a number needed to treat to benefit of 2. The same studies reported more participants achieving PASI 75 with FAE, but we did not pool the data because of significant heterogeneity; none of the studies measured PASI 90. One study reported significant improvement in participants' quality of life (QoL) with FAE, measured with Skindex-29. However, we could not compute the mean difference because of insufficient reporting in the abstract. More participants experienced adverse effects, mainly gastrointestinal disturbance and flushing, on FAE (RR 4.72, 95% CI 2.45 to 9.08; 1 study, 99 participants; moderate-quality evidence), affecting 76% of participants given FAE and 16% of the placebo group (representing a number needed to treat to harm of 2). The other studies reported similar findings or did not report adverse effects fully.One study of 54 participants compared methotrexate (MTX) with FAE. PASI score at follow-up showed superiority of MTX (mean Difference (MD) 3.80, 95% CI 0.68 to 6.92; 51 participants; very low-quality evidence), but the difference was not significant after adjustment for baseline disease severity. The difference between groups for the proportion of participants who discontinued treatment due to adverse effects was uncertain because of imprecision (RR 0.19, 95% CI 0.02 to 1.53; 1 study, 51 participants; very low-quality evidence). Overall, the number of participants experiencing common nuisance adverse effects was not significantly different between the 2 groups, with 89% of the FAE group affected compared with 100% of the MTX group (RR 0.89, 95% CI 0.77 to 1.03; 54 participants; very low-quality evidence). Flushing was more frequent in those on FAE, with 13 out of 27 participants affected compared with 2 out of 27 given MTX. There was no significant difference in the number of participants who attained PASI 50, 75, and 90 in the 2 groups (very low-quality evidence) whereas this study did not measure the effect of treatments on QoL. The included studies reported no serious adverse effects of FAE and were too small and of limited duration to provide evidence about rare or delayed effects.
Authors' conclusions: Evidence suggests that FAE are superior to placebo and possibly similar in efficacy to MTX for psoriasis; however, the evidence provided in this review was limited, and it must be noted that four out of six included studies were abstracts or brief reports, restricting study reporting. FAE are associated with nuisance adverse effects, including flushing and gastrointestinal disturbance, but short-term studies reported no serious adverse effects.
Conflict of interest statement
Ausama Atwan: nothing to declare. John R Ingram: nothing to declare. Rachel Abbott: nothing to declare. Mark J Kelson: nothing to declare. Timothy Pickles: nothing to declare. Andrea Bauer: nothing to declare. Vincent Piguet has received departmental support from AbbVie, Johnson & Johnson, Pfizer, GSK, Novartis, and CEO. He has received honoraria from Johnson & Johnson, Novartis, and AbbVie. None of these companies produce any of the interventions listed in this review. His department benefits financially from the Dermatology Life Quality Index. Ben Carter, who was the statistics referee for this review, is based at the same institution as the lead author and contributed to their MSc basic statistics teaching programme.
Figures





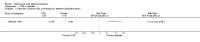


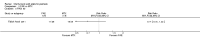

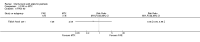

Update of
References
References to studies included in this review
Altmeyer 1994 {published data only (unpublished sought but not used)}
-
- Altmeyer PJ, Matthes U, Pawlak F, Hoffmann K, Frosch PJ, Ruppert P, et al. Antipsoriatic effect of fumaric acid derivatives. Results of a multicenter double‐blind study in 100 patients. Journal of the American Academy of Dermatology 1994;30(6):977‐81. [PUBMED: 8188891] - PubMed
Fallah Arani 2011 {published data only (unpublished sought but not used)}
-
- Fallah Arani S, Neumann H, Hop WC, Thio HB. Fumarates vs. methotrexate in moderate to severe chronic plaque psoriasis: a multicentre prospective randomized controlled clinical trial. British Journal of Dermatology 2011;164(4):855‐61. [PUBMED: 21175564] - PubMed
Langner 2004 {published data only (unpublished sought but not used)}
-
- Langner A, Roszkiewicz J, Baran E, Placek W. Results of a phase II study of a novel oral fumarate, BG‐12, in the treatment of severe psoriasis (Abstract P075). European Congress on Psoriasis 2004. Journal of the European Academy of Dermatology & Venereology 2004;18(6):798.
Mrowietz 2006 {published data only (unpublished sought but not used)}
-
- Mrowietz U, Reich K, Spellman MC. Efficacy, safety, and quality of life effects of a novel oral formulation of dimethyl fumarate in patients with moderate to severe plaque psoriasis: Results of a phase 3 study. Journal of the American Academy of Dermatology 2006;54(Suppl):AB202.
-
- Mrowietz U, Spellman M. Dimethyl Fumarate (BG00012) as an Oral Therapy for Moderate to Severe Psoriasis: Results of a Multicenter, Randomized, Double‐Blind, Placebo‐Controlled Trial (Abstract 406). 35th Annual ESDR Meeting 22‐24th September 2005, Tübingen, Germany. Journal of Investigative Dermatology 2005;125(3 Suppl):A69.
-
- Mrowietz U, Spellman MC. Results of a phase III study of a novel oral formulation of dimethylfumarate in the treatment of moderate to severe plaque psoriasis: efficacy, safety, and quality of life effects (Abstract P06.97). The 14th Congress of the European Academy of Dermatology and Venereology, London,UK. 12‐15th October 2005. Journal of the European Academy of Dermatology & Venereology : JEADV 2005;19(Suppl 2):187.
-
- Ortonne JP, Kerkhof P, Mrowietz U. A novel oral agent improves quality of life (QOL) in patients with plaque psoriasis (Abstract P013). 4th European Association of Dermatology & Venereology (EADV) Spring Symposium. Saariselka, Lapland, Finland, Feb 9th‐12th 2006. 2006:P‐013.
Nugteren‐Huying 1990 {published data only (unpublished sought but not used)}
-
- Nugteren‐Huying WM, Schroeff JG, Hermans J, Suurmond D. Fumaric acid therapy for psoriasis: A randomized, double‐blind, placebo‐controlled study. Journal of the American Academy of Dermatology 1990;22(2 Pt 1):311‐312. [PUBMED: 2312814] - PubMed
-
- Nugteren‐Huying WM, Schroeff JG, Hermans J, Suurmond D. Fumaric acid therapy in psoriasis; a double‐blind, placebo‐controlled study [Fumaarzuurtherapie tegen psoriasis; een dubbelblind, placebo‐gecontroleerd onderzoek]. Nederlands Tijdschrift Voor Geneeskunde 1990;134(49):2387‐91. [PUBMED: 2263264] - PubMed
Peeters 1992 {published data only (unpublished sought but not used)}
-
- Peeters AJ, Dijkmans BA, Schroeff JG. Fumaric acid therapy for psoriatic arthritis. A randomized, double‐blind, placebo‐controlled study. British Journal of Rheumatology 1992;31(7):502‐4. [PUBMED: 1628175] - PubMed
-
- Peeters AJ, Dijmans BAC, Schroeff JG. Favourable effect of fumaric acid treatment in psoriatic arthritis: A double‐blind placebo‐controlled study [Gunstig effect van fumaarzuurtherapie bij arthritis psoriatica: een dubbelblind, placebo‐gecontroleerd onderzoek]. Nederlands Tijdschrift Voor Geneeskunde 1992;136(49):2428‐31. [EMBASE: 1993004024]
References to studies excluded from this review
Balak 2015 {published data only}
-
- Balak DM, Fallah‐Arani S, Venema CM, Neumann HA, Thio HB. Addition of an oral histamine antagonist to reduce adverse events associated with fumaric acid esters in the treatment of psoriasis: a randomized double‐blind placebo‐controlled trial. British Journal of Dermatology 2015;172(3):754‐9. [PUBMED: 25041291] - PubMed
Friedrich 2001 {published data only}
-
- Friedrich M, Sterry W, Klein A, Rückert R, Döcke WD, Asadullah K. Addition of pentoxifylline could reduce the side effects of fumaric acid esters in the treatment of psoriasis. Acta Dermato‐Venereologica 2001;81(6):429‐30. [PUBMED: 11859949] - PubMed
Gollnick 2002 {published data only}
-
- Gollnick H, Altmeyer P, Kaufmann R, Ring J, Christophers E, Pavel S, et al. Topical calcipotriol plus oral fumaric acid is more effective and faster acting than oral fumaric acid monotherapy in the treatment of severe chronic plaque psoriasis vulgaris. Dermatology 2002;205(1):46‐53. [PUBMED: 12145434] - PubMed
Nieboer 1989 {published data only (unpublished sought but not used)}
-
- Nieboer C, Hoop D, Loenen AC, Langendijk PNJ, Dijk E. Systemic therapy with fumaric acid derivates: New possibilities in the treatment of psoriasis. Journal of the American Academy of Dermatology 1989;20(4):601‐8. [PUBMED: 2654206] - PubMed
Nieboer 1990 {published data only}
-
- Nieboer C, Hoop D, Langendijk PN, Loenen AC, Gubbels J. Fumaric acid therapy in psoriasis: a double‐blind comparison between fumaric acid compound therapy and monotherapy with dimethylfumaric acid ester. Dermatologica 1990;181(1):33‐7. [PUBMED: 2394301] - PubMed
References to ongoing studies
DRKS00000716 {unpublished data only}
-
- DRKS00000716. Regulatory T‐cell function in psoriasis vulgaris. apps.who.int/trialsearch/Trial2.aspx?TrialID=DRKS00000716 (accessed 14 May 2015).
EudraCT Number 2012‐000035‐82 {unpublished data only}
-
- EudraCT Number: 2012‐000035‐82. A 2:1 randomized, double‐blinded, placebo‐controlled study to evaluate the efficacy and safety of Fumaderm® in young patients aged 10 to 17 years with moderate to severe psoriasis vulgaris (KIFUderm study). www.clinicaltrialsregister.eu/ctr‐search/trial/2012‐000035‐82/DE (accessed 14 May 2015).
EudraCT Number 2012‐000055‐13 {unpublished data only}
-
- EudraCT Number: 2012‐000055‐13. A multi‐center, randomized, double‐blind, three‐arm, 16 week, adaptive phase III clinical study to investigate the efficacy and safety of LAS41008 vs LASW1835 and vs Placebo in patients with moderate to severe plaque psoriasis. www.clinicaltrialsregister.eu/ctr‐search/trial/2012‐000055‐13/AT (accessed 14 May 2015).
EudraCT Number 2012‐005685‐35 {unpublished data only}
-
- EudraCT Number: 2012‐005685‐35. A randomised, double blind, double dummy, active comparator and placebo controlled confirmative non‐inferiority trial of FP187 compared to Fumaderm in moderate to severe plaque psoriasis. www.clinicaltrialsregister.eu/ctr‐search/search?query=eudract_number%3A2... (accessed 14 May 2015).
EudraCT Number 2014‐005258‐20 {unpublished data only}
-
- EudraCT Number: 2014‐005258‐20. A 24‐week, randomized, controlled, multicenter, open‐label study with blinded assessment of the efficacy of subcutaneous secukinumab compared to Fumaderm® in adults with moderate to severe plaque psoriasis. www.clinicaltrialsregister.eu/ctr‐search/trial/2014‐005258‐20/DE (accessed 14 May 2015).
NCT00811005 {unpublished data only}
-
- NCT00811005. Fumaric Acid Ester‐PUVA Therapy Versus Acitretin ‐PUVA Therapy in Pustular Palmoplantar Psoriasis (FVSA‐PUVA). www.clinicaltrials.gov/show/NCT00811005 (accessed 14 May 2015).
NCT01088165 {unpublished data only}
-
- NCT01088165. The Influence of Adalimumab on Cardiovascular and Metabolic Risk in Psoriasis (CASTIP). www.clinicaltrials.gov/show/NCT01088165 (accessed 14 May 2015).
NCT01321164 {unpublished data only}
-
- NCT01321164. Fumaric Acid Versus Fumaric Acid Plus Narrow Band Type B Ultraviolet (UVB) for Psoriasis. www.clinicaltrials.gov/ct2/show/NCT01321164 (accessed 14 May 2015).
Additional references
Augustin 2010
-
- Augustin M, Ogilvie A. Methods of outcomes measurement in nail psoriasis. Dermatology 2010;221(Suppl 1):23‐8. [MEDLINE: ] - PubMed
Baker 1984
-
- Baker BS, Swain AF, Fry L, Valdimarsson H. Epidermal T lymphocytes and HLA‐DR expression in psoriasis. British Journal of Dermatology 1984;110(5):555‐64. [PUBMED: 6232938] - PubMed
Balak 2016
-
- Balak DM, Fallah Arani S, Hajdarbegovic E. Efficacy, effectiveness and safety of fumaric acid esters in the treatment of psoriasis: a systematic review of randomized and observational studies. British Journal of Dermatology 2016;175:250‐62. [PUBMED: 26919824] - PubMed
Basavaraj 2010
-
- Basavaraj KH, Ashok NM, Rashmi R, Praveen TK. The role of drugs in the induction and/or exacerbation of psoriasis. International Journal of Dermatology 2010;49(12):1351‐61. [MEDLINE: ] - PubMed
Bovenschen 2010
-
- Bovenschen HJ, Langewouters AM, Kerkhof PC. Dimethylfumarate for psoriasis: Pronounced effects on lesional T‐cell subsets, epidermal proliferation and differentiation, but not on natural killer T cells in immunohistochemical study. American Journal of Clinical Dermatology 2010;11(5):343‐50. [MEDLINE: ] - PubMed
Burden 2012
-
- Burden AD, Warren RB, Kleyn CE, McElhone K, Smith CH, Reynolds NJ, et al. The British Association of Dermatologists' Biologic Interventions Register (BADBIR): design, methodology and objectives. British Journal of Dermatology 2012;166(3):545‐54. [MEDLINE: ] - PubMed
Carboni 2004
-
- Carboni I, Felice C, Simoni I, Soda R, Chimenti S. Fumaric acid esters in the treatment of psoriasis: an Italian experience. Journal of Dermatological Treatment 2004;15(1):23‐6. [PUBMED: 14754645] - PubMed
Ceglowska 2014
-
- Ceglowska U, Wlodarczyk A, Slomka M. Clinical effectiveness of fumaric acid esters (Fumaderm) in psoriasis: A systematic review of literature. ISPOR 17th Annual European Congress. Amsterdam, The Netherlands, Nov 2014:PSS6. - PubMed
Duffy 1993
-
- Duffy DL, Spelman LS, Martin NG. Psoriasis in Australian twins. Journal of the American Academy of Dermatology 1993;29(3):428‐34. [MEDLINE: ] - PubMed
Eedy 1990
Egger 1997
Eghlileb 2007
-
- Eghlileb AM, Davies EE, Finlay AY. Psoriasis has a major secondary impact on the lives of family members and partners. British Journal of Dermatology 2007;156(6):1245‐50. [MEDLINE: ] - PubMed
García‐Caballero 2011
-
- García‐Caballero M, Marí‐Beffa M, Medina MÁ, Quesada AR. Dimethylfumarate inhibits angiogenesis in vitro and in vivo: a possible role for its antipsoriatic effect?. Journal of Investigative Dermatology 2011;131(6):1347‐55. [MEDLINE: ] - PubMed
Ghoreschi 2011
Griffiths 2000
Griffiths 2007
-
- Griffiths CE, Barker JN. Pathogenesis and clinical features of psoriasis. Lancet 2007;370(9583):263–71. [MEDLINE: ] - PubMed
Harries 2005
-
- Harries MJ, Chalmers RJ, Griffiths CE. Fumaric acid esters for severe psoriasis: a retrospective review of 58 cases. British Journal of Dermatology 2005;153(3):549‐51. [PUBMED: 16120141] - PubMed
Heidenreich 2009
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration 2011. Available from www.cochrane‐handbook.org.
Hoefnagel 2003
-
- Hoefnagel JJ, Thio HB, Willemze R, Bouwes Bavinck JN. Long‐term safety aspects of systemic therapy with fumaric acid esters in severe psoriasis. British Journal of Dermatology 2003;149(2):363‐9. [MEDLINE: ] - PubMed
Huerta 2007
-
- Huerta C, Rivero E, Rodriguez LA. Incidence and risk factors for psoriasis in the general population. Archives of Dermatology 2007;143(12):1559‐65. [MEDLINE: ] - PubMed
Ingram 2012
Kimball 2005
-
- Kimball AB, Jacobson C, Weiss S, Vreeland MG, Wu Y. The psychosocial burden of psoriasis. American Journal of Clinical Dermatology 2005;6(6):383‐92. [MEDLINE: ] - PubMed
Kokelj 2009
-
- Kokelj F, Plozzer C, Avian A, Trevisan G. Fumaric acid and its derivatives in the treatment of psoriasis vulgaris: our experience in forty‐one patients. Acta Dermatovenerologica Croatica 2009;17(3):170‐5. [PUBMED: 19818215] - PubMed
Langley 2005
Lebwohl 2003
-
- Lebwohl M. Psoriasis. Lancet 2003;361(9364):1197‐204. [MEDLINE: ] - PubMed
Mehta 2010
Meissner 2011
-
- Meissner M, Doll M, Hrgovic I, Reichenbach G, König V, Hailemariam‐Jahn T, et al. Suppression of VEGFR2 expression in human endothelial cells by dimethylfumarate treatment: evidence for anti‐angiogenic action. Journal of Investigative Dermatology 2011;131(6):1356‐64. - PubMed
Menter 2007
-
- Menter A, Griffiths CEM. Current and future management of psoriasis. Lancet 2007;370(9583):272‐84. [PUBMED: 17658398] - PubMed
Mrowietz 1999
-
- Mrowietz U, Christophers E, Altmeyer P. Treatment of severe psoriasis with fumaric acid esters: scientific background and guidelines for therapeutic use. The German Fumaric Acid Ester Consensus Conference. British Journal of Dermatology 1999;141(3):424‐29. [MEDLINE: ] - PubMed
Mrowietz 2005
-
- Mrowietz U, Asadullah K. Dimethylfumarate for psoriasis: more than a dietary curiosity. Trends in Molecular Medicine 2005;11(1):43‐8. [PUBMED: 15649822] - PubMed
Mustafa 2013
-
- Mustafa AA, Al‐Hoqail IA. Biologic systemic therapy for moderate‐to‐severe psoriasis: A review. Journal of Taibah University Medical Sciences 2013;8(3):142‐50. [DOI: 10.1016/j.jtumed.2013.09.001] - DOI
Nast 2012
-
- Nast A, Boehncke WH, Mrowietz U, Ockenfels HM, Philipp S, Reich K, et al. S3 ‐ Guidelines on the treatment of psoriasis vulgaris (English version). Update. Journal der Deutschen Dermatologischen Gesellschaft 2012;10(Suppl 2):S1‐95. [MEDLINE: ] - PubMed
Nestle 2009
-
- Nestle FO, Kaplan DH, Barker J. Psoriasis. New England Journal of Medicine 2009;361(5):496‐509. [MEDLINE: ] - PubMed
O'Rourke 1989
-
- O'Rourke K, Detsky AS. Meta‐analysis in medical research: strong encouragement for higher quality in individual research efforts. Journal of Clinical Epidemiology 1989;42(10):1021‐4. [MEDLINE: ] - PubMed
Onderdijk 2014
-
- Onderdijk AJ, Balak DM, Baerveldt EM, Florencia EF, Kant M, Laman JD, et al. Regulated genes in psoriatic skin during treatment with fumaric acid esters. British Journal of Dermatology 2014;171(4):732‐41. [MEDLINE: ] - PubMed
Parisi 2012
-
- Parisi R, Symmons DP, Griffiths CE, Ashcroft DM, on behalf of the Identification and Management of Psoriasis and Associated ComorbidiTy (IMPACT) project team. Global Epidemiology of Psoriasis: A Systematic Review of Incidence and Prevalence. Journal of Investigative Dermatology 2013;133(2):377‐85. [DOI: 10.1038/jid.2012.339; MEDLINE: ] - DOI - PubMed
Pathirana 2009
-
- Pathirana D, Ormerod AD, Saiag P, Smith C, Spuls PI, Nast A, et al. European S3‐Guidelines on the systemic treatment of psoriasis vulgaris. Journal of the European Academy of Dermatology & Venereology 2009;23(Suppl 2):1‐70. [MEDLINE: ] - PubMed
Rapp 1999
-
- Rapp SR, Feldman SR, Exum ML, Fleischer AB Jr, Reboussin DM. Psoriasis causes as much disability as other major medical diseases. Journal of the American Academy of Dermatology 1999;41(3 Pt 1):401‐7. [MEDLINE: ] - PubMed
Rubant 2008
-
- Rubant SA, Ludwig RJ, Diehl S, Hardt K, Kaufmann R, Pfeilschifter JM, et al. Dimethylfumarate reduces leukocyte rolling in vivo through modulation of adhesion molecule expression. Journal of Investigative Dermatology 2008;128(2):326‐31. [MEDLINE: ] - PubMed
Schmitt 2014
-
- Schmitt J, Rosumeck S, Thomaschewski G, Sporbeck B, Haufe E, Nast A. Efficacy and safety of systemic treatments for moderate‐to‐severe psoriasis: meta‐analysis of randomized controlled trials. British Journal of Dermatology 2014;170(2):274‐303. [MEDLINE: ] - PubMed
Sladden 2006
-
- Sladden MJ, Osborne JE, Hutchinson PE. Fumaric acid esters for severe psoriasis: the Leicestershire experience. British Journal of Dermatology 2006;155(4):843‐4. [PUBMED: 16965442] - PubMed
Smith 2006
Smith 2009
-
- Smith CH, Anstey AV, Barker JN, Burden AD, Chalmers RJ, Chandler DA, et al. British Association of Dermatologists' guidelines for biologic interventions for psoriasis 2009. British Journal of Dermatology 2009;161(5):987‐1019. [MEDLINE: ] - PubMed
Taylor 2006
-
- Taylor W, Gladman D, Helliwell P, Marchesoni A, Mease P, Mielants H, et al. Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis & Rheumatology 2006;54(8):2665‐73. [PUBMED: 16871531] - PubMed
Telfer 1992
-
- Telfer NR, Chalmers RJ, Whale K, Colman G. The role of streptococcal infection in the initiation of guttate psoriasis. Archives of Dermatology 1992;128(1):39‐42. [MEDLINE: ] - PubMed
Trembath 1997
-
- Trembath RC, Clough RL, Rosbotham JL, Jones AB, Camp RD, Frodsham A, et al. Identification of a major susceptibility locus on chromosome 6p and evidence for further disease loci revealed by a two stage genome‐wide search in psoriasis. Human Molecular Genetics 1997;6(5):813‐20. [PUBMED: 9158158] - PubMed
Walker 2014
-
- Walker F, Adamczyk A, Kellerer C, Belge K, Bruck J, Berner T, et al. Fumaderm® in daily practice for psoriasis: dosing, efficacy and quality of life. British Journal of Dermatology 2014;171(5):1197‐1205. [MEDLINE: ] - PubMed
Warren 2011
-
- Warren RB, Kleyn CE, Gulliver WP. Cumulative life course impairment in psoriasis: patient perception of disease‐related impairment throughout the life course. British Journal of Dermatology 2011;164(Suppl 1):1‐14. [MEDLINE: ] - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

