Advances in Diagnosis and Treatment of Fetal Alcohol Spectrum Disorders: From Animal Models to Human Studies
- PMID: 26259091
- PMCID: PMC4476607
Advances in Diagnosis and Treatment of Fetal Alcohol Spectrum Disorders: From Animal Models to Human Studies
Abstract
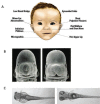
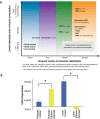
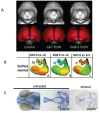
Prenatal alcohol exposure can cause a number of physical, behavioral, cognitive, and neural impairments, collectively known as fetal alcohol spectrum disorders (FASD). This article examines basic research that has been or could be translated into practical applications for the diagnosis or treatment of FASD. Diagnosing FASD continues to be a challenge, but advances are being made at both basic science and clinical levels. These include identification of biomarkers, recognition of subtle facial characteristics of exposure, and examination of the relation between face, brain, and behavior. Basic research also is pointing toward potential new interventions for FASD involving pharmacotherapies, nutritional therapies, and exercise interventions. Although researchers have assessed the majority of these treatments in animal models of FASD, a limited number of recent clinical studies exist. An assessment of this literature suggests that targeted interventions can improve some impairments resulting from developmental alcohol exposure. However, combining interventions may prove more efficacious. Ultimately, advances in basic and clinical sciences may translate to clinical care, improving both diagnosis and treatment.
Figures


References
-
- Bekdash RA, Zhang C, Sarkar DK. Gestational choline supplementation normalized fetal alcohol-induced alterations in histone modifications, DNA methylation, and proopiomelanocortin (POMC) gene expression in β-endorphin-producing POMC neurons of the hypothalamus. Alcoholism: Clinical and Experimental Research. 2013;37(7):1133–1142. - PMC - PubMed
-
- Bertrand J, Floyd LL, Weber MK. Guidelines for identifying and referring persons with fetal alcohol syndrome. Morbidity and Mortality Weekly Report (MMWR) Recommendations and Reports. 2005;54(RR–11):1–14. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

