The intracellular domain of cell adhesion molecule 1 is present in emphysematous lungs and induces lung epithelial cell apoptosis
- PMID: 26259600
- PMCID: PMC4531499
- DOI: 10.1186/s12929-015-0173-8
The intracellular domain of cell adhesion molecule 1 is present in emphysematous lungs and induces lung epithelial cell apoptosis
Abstract
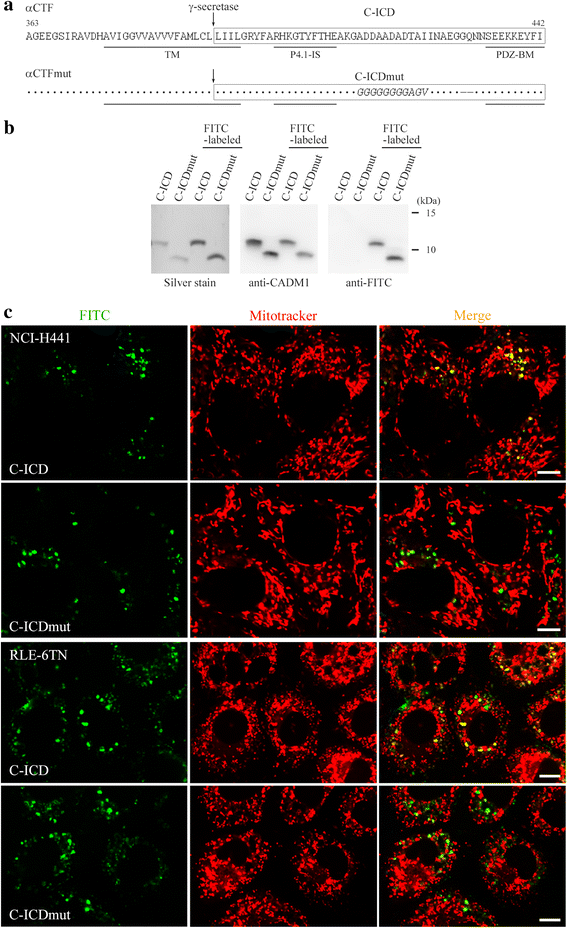
Background: Pulmonary emphysema is characterized histologically by destruction of alveolar walls and enlargement of air spaces due to lung epithelial cell apoptosis. Cell adhesion molecule 1 (CADM1) is an immunoglobulin superfamily member expressed in lung epithelial cells. CADM1 generates a membrane-associated C-terminal fragment, αCTF, through A disintegrin- and metalloprotease-10-mediated ectodomain shedding, subsequently releasing the intracellular domain (ICD) through γ-secretase-mediated intramembrane shedding of αCTF. αCTF localizes to mitochondria and induces apoptosis in lung epithelial cells. αCTF contributes to the development and progression of emphysema as a consequence of increased CADM1 ectodomain shedding. The purpose of this study was to examine whether the ICD makes a similar contribution.
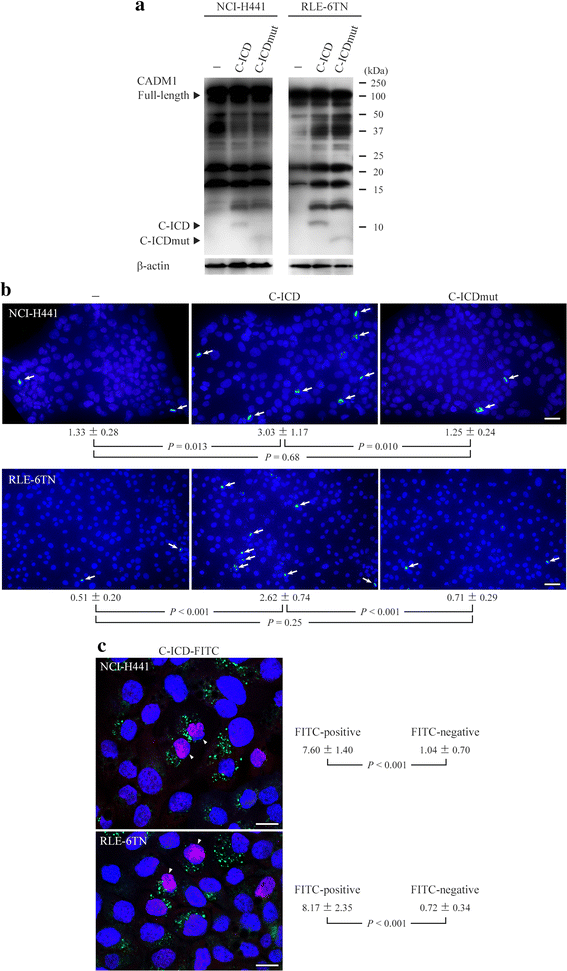
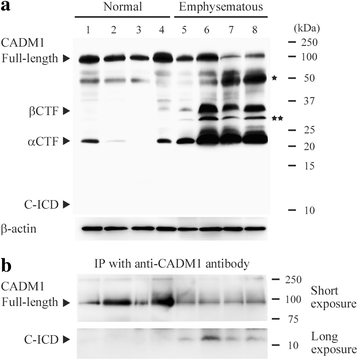
Results: The ICD was synthesized as a 51-amino acid peptide, and its mutant was synthesized by substituting seven amino acids and deleting two amino acids. These peptides were labeled with fluorescein isothiocyanate and were introduced into various cell lines. ICD peptide-derived fluorescence was well visualized in lung epithelial cells at the site of Mitotracker mitochondrial labeling, but was detected in locations other than mitochondria in other cell types. Mutant peptide-derived fluorescence was detected in locations other than mitochondria, even in lung epithelial cells. Terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling assays revealed that transduction of the ICD peptide increased the proportion of apoptotic cells 2- to 5-fold in the lung epithelial cell lines, whereas the mutant peptide did not. Abundance of the ICD was below the Western blot detection limit in emphysematous (n = 4) and control (n = 4) human lungs. However, the ICD was detected only in emphysematous lungs when it was immunoprecipitated with anti-CADM1 antibody (4/4 vs. 0/4, P = 0.029).
Conclusions: As the abundance of ICD molecules was sparse but present, increased CADM1 shedding appeared to contribute to the development of emphysema by generating αCTF and the ICD in lung epithelial cells.
Figures

Similar articles
-
Pathogenic Actions of Cell Adhesion Molecule 1 in Pulmonary Emphysema and Atopic Dermatitis.Front Cell Dev Biol. 2015 Nov 20;3:75. doi: 10.3389/fcell.2015.00075. eCollection 2015. Front Cell Dev Biol. 2015. PMID: 26636084 Free PMC article. Review.
-
Increased ectodomain shedding of cell adhesion molecule 1 as a cause of type II alveolar epithelial cell apoptosis in patients with idiopathic interstitial pneumonia.Respir Res. 2015 Aug 1;16:90. doi: 10.1186/s12931-015-0255-x. Respir Res. 2015. PMID: 26231557 Free PMC article.
-
Increased ectodomain shedding of lung epithelial cell adhesion molecule 1 as a cause of increased alveolar cell apoptosis in emphysema.Thorax. 2014 Mar;69(3):223-31. doi: 10.1136/thoraxjnl-2013-203867. Epub 2013 Oct 2. Thorax. 2014. PMID: 24092566 Free PMC article.
-
Increased ectodomain shedding of cell adhesion molecule 1 from pancreatic islets in type 2 diabetic pancreata: correlation with hemoglobin A1c levels.PLoS One. 2014 Jun 25;9(6):e100988. doi: 10.1371/journal.pone.0100988. eCollection 2014. PLoS One. 2014. PMID: 24964098 Free PMC article.
-
Alveolar epithelial and endothelial cell apoptosis in emphysema: what we know and what we need to know.Int J Chron Obstruct Pulmon Dis. 2009;4:19-31. Epub 2009 Apr 15. Int J Chron Obstruct Pulmon Dis. 2009. PMID: 19436685 Free PMC article. Review.
Cited by
-
Pathogenic Actions of Cell Adhesion Molecule 1 in Pulmonary Emphysema and Atopic Dermatitis.Front Cell Dev Biol. 2015 Nov 20;3:75. doi: 10.3389/fcell.2015.00075. eCollection 2015. Front Cell Dev Biol. 2015. PMID: 26636084 Free PMC article. Review.
-
Pathological observation of the effects of exposure to radioactive microparticles on experimental animals.J Radiat Res. 2022 Aug 13;63(Supplement_1):i26-i37. doi: 10.1093/jrr/rrac045. J Radiat Res. 2022. PMID: 35968993 Free PMC article. Review.
-
Cell Adhesion Molecule 1 Contributes to Cell Survival in Crowded Epithelial Monolayers.Int J Mol Sci. 2020 Jun 9;21(11):4123. doi: 10.3390/ijms21114123. Int J Mol Sci. 2020. PMID: 32527032 Free PMC article.
-
Somatic mutations of CADM1 in aldosterone-producing adenomas and gap junction-dependent regulation of aldosterone production.Nat Genet. 2023 Jun;55(6):1009-1021. doi: 10.1038/s41588-023-01403-0. Epub 2023 Jun 8. Nat Genet. 2023. PMID: 37291193 Free PMC article.
-
Impact of Local High Doses of Radiation by Neutron Activated Mn Dioxide Powder in Rat Lungs: Protracted Pathologic Damage Initiated by Internal Exposure.Biomedicines. 2020 Jun 23;8(6):171. doi: 10.3390/biomedicines8060171. Biomedicines. 2020. PMID: 32586004 Free PMC article.
References
-
- Snider GL, Kleinerman J, Thurlbeck WM, Bengali ZH. The definition of emphysema. Report of a National Heart, Lung, and Blood Institute, Division of Lung Diseases workshop. Am Rev Respir Dis. 1985;132:182–5. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

