Highly Pathogenic Avian Influenza A(H5N1) Virus Struck Migratory Birds in China in 2015
- PMID: 26259704
- PMCID: PMC4531313
- DOI: 10.1038/srep12986
Highly Pathogenic Avian Influenza A(H5N1) Virus Struck Migratory Birds in China in 2015
Abstract
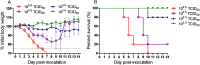
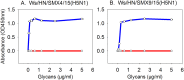
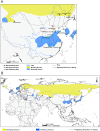
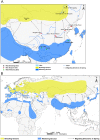
Approximately 100 migratory birds, including whooper swans and pochards, were found dead in the Sanmenxia Reservoir Area of China during January 2015. The causative agent behind this outbreak was identified as H5N1 highly pathogenic avian influenza virus (HPAIV). Genetic and phylogenetic analyses revealed that this Sanmenxia H5N1 virus was a novel reassortant, possessing a Clade 2.3.2.1c HA gene and a H9N2-derived PB2 gene. Sanmenxia Clade 2.3.2.1c-like H5N1 viruses possess the closest genetic identity to A/Alberta/01/2014 (H5N1), which recently caused a fatal respiratory infection in Canada with signs of meningoencephalitis, a highly unusual symptom with influenza infections in humans. Furthermore, this virus was shown to be highly pathogenic to both birds and mammals, and demonstrate tropism for the nervous system. Due to the geographical location of Sanmenxia, these novel H5N1 viruses also have the potential to be imported to other regions through the migration of wild birds, similar to the H5N1 outbreak amongst migratory birds in Qinghai Lake during 2005. Therefore, further investigation and monitoring is required to prevent this novel reassortant virus from becoming a new threat to public health.
Figures


Similar articles
-
Multiple introductions of a reassortant H5N1 avian influenza virus of clade 2.3.2.1c with PB2 gene of H9N2 subtype into Indian poultry.Infect Genet Evol. 2016 Sep;43:173-8. doi: 10.1016/j.meegid.2016.05.012. Epub 2016 May 10. Infect Genet Evol. 2016. PMID: 27174088
-
Genetic analysis of avian influenza A viruses isolated from domestic waterfowl in live-bird markets of Hanoi, Vietnam, preceding fatal H5N1 human infections in 2004.Arch Virol. 2009;154(8):1249-61. doi: 10.1007/s00705-009-0429-2. Epub 2009 Jul 4. Arch Virol. 2009. PMID: 19578928
-
Clade 2.3.2.1 H5N1 avian influenza viruses circulate at the interface of migratory and domestic birds around Qinghai Lake in China.Vet Microbiol. 2019 Aug;235:234-242. doi: 10.1016/j.vetmic.2019.07.009. Epub 2019 Jul 11. Vet Microbiol. 2019. PMID: 31383307
-
Global patterns of influenza a virus in wild birds.Science. 2006 Apr 21;312(5772):384-8. doi: 10.1126/science.1122438. Science. 2006. PMID: 16627734 Review.
-
H5N1 influenza viruses: outbreaks and biological properties.Cell Res. 2010 Jan;20(1):51-61. doi: 10.1038/cr.2009.124. Epub 2009 Nov 3. Cell Res. 2010. PMID: 19884910 Free PMC article. Review.
Cited by
-
Avian influenza.EFSA J. 2017 Oct 16;15(10):e04991. doi: 10.2903/j.efsa.2017.4991. eCollection 2017 Oct. EFSA J. 2017. PMID: 32625288 Free PMC article.
-
Zoonotic Potential of Influenza A Viruses: A Comprehensive Overview.Viruses. 2018 Sep 13;10(9):497. doi: 10.3390/v10090497. Viruses. 2018. PMID: 30217093 Free PMC article. Review.
-
Southward autumn migration of waterfowl facilitates cross-continental transmission of the highly pathogenic avian influenza H5N1 virus.Sci Rep. 2016 Aug 10;6:30262. doi: 10.1038/srep30262. Sci Rep. 2016. PMID: 27507581 Free PMC article.
-
New evidence for the east-west spread of the highly pathogenic avian influenza H5N1 virus between Central Asian and east Asian-Australasian flyways in China.Emerg Microbes Infect. 2019;8(1):823-826. doi: 10.1080/22221751.2019.1623719. Emerg Microbes Infect. 2019. PMID: 31164049 Free PMC article.
-
Genetic and Pathogenic Characterization of Avian Influenza Virus in Migratory Birds between 2015 and 2019 in Central China.Microbiol Spectr. 2022 Aug 31;10(4):e0165222. doi: 10.1128/spectrum.01652-22. Epub 2022 Jul 12. Microbiol Spectr. 2022. PMID: 35862978 Free PMC article.
References
-
- WHO. Cumulative number of confirmed human cases for avian influenza A(H5N1) reported to WHO, 2003–2015. (2015) Available at: http://www.who.int/influenza/human_animal_interface/H5N1_cumulative_tabl.... (Accessed: 31th March 2015).
-
- WHO. Monthly Risk Assessment Summary, Influenza at the Human-Animal Interface. (2015) Available at: http://www.who.int/influenza/human_animal_interface/HAI_Risk_Assessment/en/. (Accessed: 31th March 2015).
-
- OIE. Update on highly pathogenic avian influenza in animals (Type H5 and H7). (2015) Available at: http://www.oie.int/en/animal-health-in-the-world/update-on-avian-influen.... (Accessed: 31th March 2015).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

