Comparative Genomic Analysis of Meningitis- and Bacteremia-Causing Pneumococci Identifies a Common Core Genome
- PMID: 26259813
- PMCID: PMC4567637
- DOI: 10.1128/IAI.00814-15
Comparative Genomic Analysis of Meningitis- and Bacteremia-Causing Pneumococci Identifies a Common Core Genome
Erratum in
-
Erratum for Kulohoma et al., Comparative genomic analysis of meningitis- and bacteremia-causing pneumococci identifies a common core genome.Infect Immun. 2015 Dec;83(12):4896. doi: 10.1128/IAI.01156-15. Infect Immun. 2015. PMID: 26556879 Free PMC article. No abstract available.
Abstract
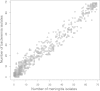
Streptococcus pneumoniae is a nasopharyngeal commensal that occasionally invades normally sterile sites to cause bloodstream infection and meningitis. Although the pneumococcal population structure and evolutionary genetics are well defined, it is not clear whether pneumococci that cause meningitis are genetically distinct from those that do not. Here, we used whole-genome sequencing of 140 isolates of S. pneumoniae recovered from bloodstream infection (n = 70) and meningitis (n = 70) to compare their genetic contents. By fitting a double-exponential decaying-function model, we show that these isolates share a core of 1,427 genes (95% confidence interval [CI], 1,425 to 1,435 genes) and that there is no difference in the core genome or accessory gene content from these disease manifestations. Gene presence/absence alone therefore does not explain the virulence behavior of pneumococci that reach the meninges. Our analysis, however, supports the requirement of a range of previously described virulence factors and vaccine candidates for both meningitis- and bacteremia-causing pneumococci. This high-resolution view suggests that, despite considerable competency for genetic exchange, all pneumococci are under considerable pressure to retain key components advantageous for colonization and transmission and that these components are essential for access to and survival in sterile sites.
Copyright © 2015 Kulohoma et al.
Figures


References
-
- Orihuela CJ, Mahdavi J, Thornton J, Mann B, Wooldridge KG, Abouseada N, Oldfield NJ, Self T, Ala'Aldeen DA, Tuomanen EI. 2009. Laminin receptor initiates bacterial contact with the blood brain barrier in experimental meningitis models. J Clin Invest 119:1638–1646. doi:10.1172/JCI36759. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

