A Quantitative Approach to Screen for Nephrotoxic Compounds In Vitro
- PMID: 26260164
- PMCID: PMC4814182
- DOI: 10.1681/ASN.2015010060
A Quantitative Approach to Screen for Nephrotoxic Compounds In Vitro
Abstract
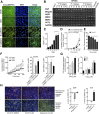
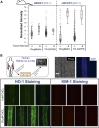
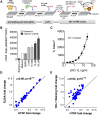
Nephrotoxicity due to drugs and environmental chemicals accounts for significant patient mortality and morbidity, but there is no high throughput in vitro method for predictive nephrotoxicity assessment. We show that primary human proximal tubular epithelial cells (HPTECs) possess characteristics of differentiated epithelial cells rendering them desirable to use in such in vitro systems. To identify a reliable biomarker of nephrotoxicity, we conducted multiplexed gene expression profiling of HPTECs after exposure to six different concentrations of nine human nephrotoxicants. Only overexpression of the gene encoding heme oxygenase-1 (HO-1) significantly correlated with increasing dose for six of the compounds, and significant HO-1 protein deregulation was confirmed with each of the nine nephrotoxicants. Translatability of HO-1 increase across species and platforms was demonstrated by computationally mining two large rat toxicogenomic databases for kidney tubular toxicity and by observing a significant increase in HO-1 after toxicity using an ex vivo three-dimensional microphysiologic system (kidney-on-a-chip). The predictive potential of HO-1 was tested using an additional panel of 39 mechanistically distinct nephrotoxic compounds. Although HO-1 performed better (area under the curve receiver-operator characteristic curve [AUC-ROC]=0.89) than traditional endpoints of cell viability (AUC-ROC for ATP=0.78; AUC-ROC for cell count=0.88), the combination of HO-1 and cell count further improved the predictive ability (AUC-ROC=0.92). We also developed and optimized a homogenous time-resolved fluorescence assay to allow high throughput quantitative screening of nephrotoxic compounds using HO-1 as a sensitive biomarker. This cell-based approach may facilitate rapid assessment of potential nephrotoxic therapeutics and environmental chemicals.
Keywords: acute renal failure; nephrotoxicity; tubule cells.
Copyright © 2016 by the American Society of Nephrology.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

