A critical role of Oct4A in mediating metastasis and disease-free survival in a mouse model of ovarian cancer
- PMID: 26260289
- PMCID: PMC4531496
- DOI: 10.1186/s12943-015-0417-y
A critical role of Oct4A in mediating metastasis and disease-free survival in a mouse model of ovarian cancer
Abstract
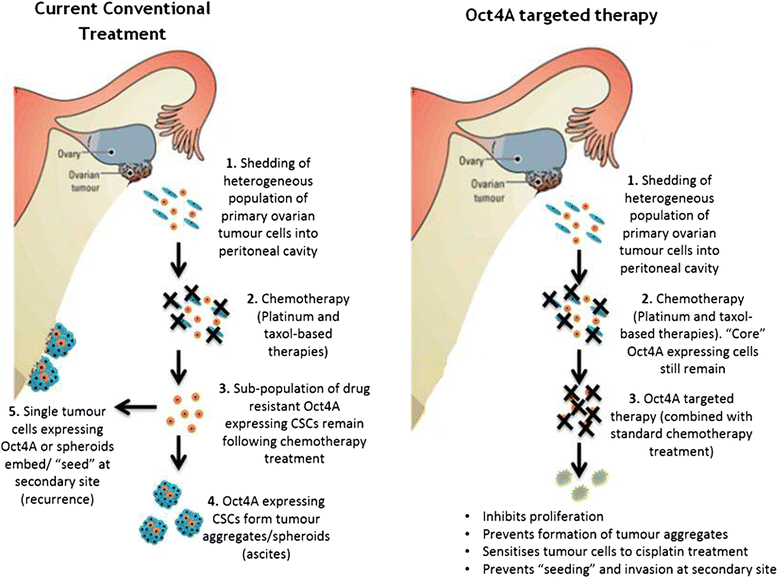
Background: High grade epithelial ovarian cancer (EOC) is commonly characterised by widespread peritoneal dissemination and ascites. Metastatic EOC tumour cells can attach directly to neighbouring organs or alternatively, maintain long term tumourigenicity and chemoresistance by forming cellular aggregates (spheroids). Cancer stem-like cells are proposed to facilitate this mechanism. This study aimed to investigate the role of Oct4A, an embryonic stem cell factor and known master regulator of pluripotency in EOC progression, metastasis and chemoresistance.
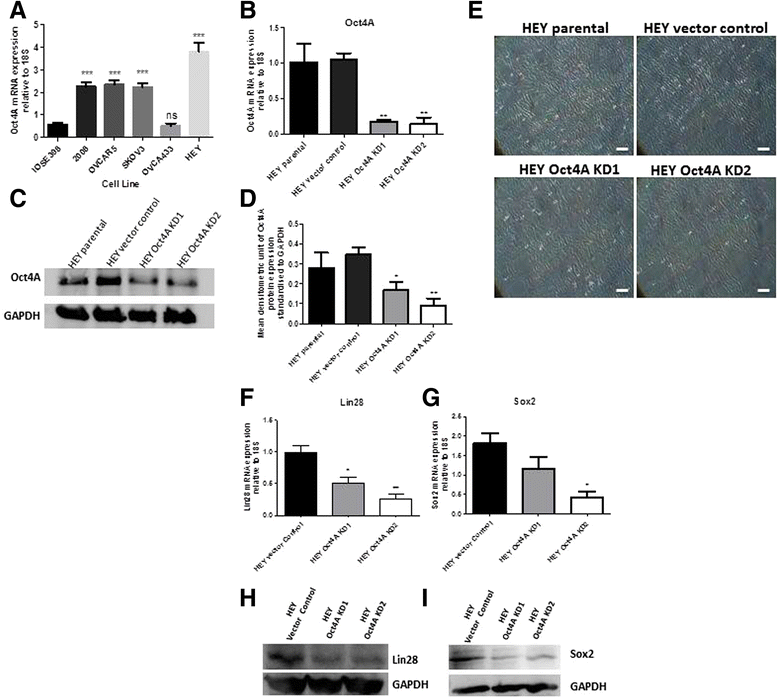
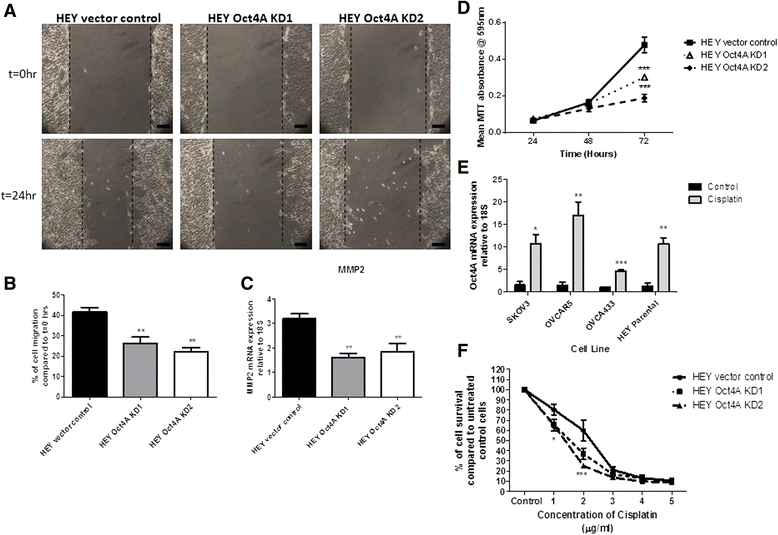
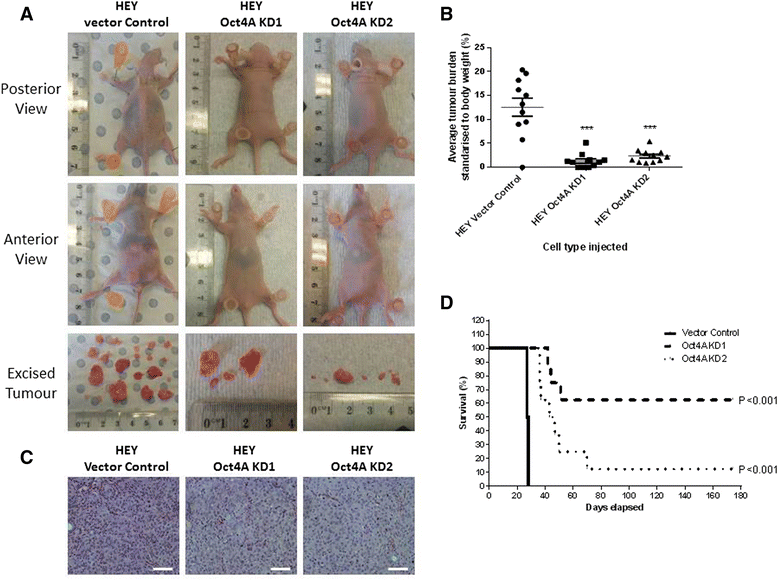
Methods: To investigate the expression of Oct4A in primary EOC tumours, IHC and qRT-PCR analyses were used. The expression of Oct4A in chemonaive and recurrent EOC patient ascites-derived tumour cells samples was investigated by qRT-PCR. The functional role of Oct4A in EOC was evaluated by generating stable knockdown Oct4A clones in the established EOC cell line HEY using shRNA-mediated silencing technology. Cellular proliferation, spheroid forming ability, migration and chemosensitivty following loss of Oct4A in HEY cells was measured by in vitro functional assays. These observations were further validated in an in vivo mouse model using intraperitoneal (IP) injection of established Oct4A KD clones into Balb/c nu/nu mice.
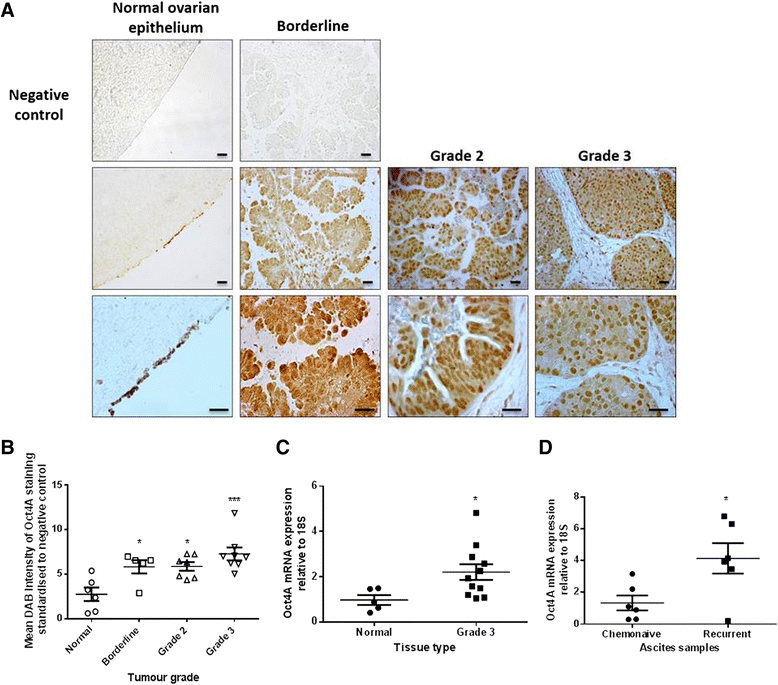
Results: We demonstrate that, compared to normal ovaries Oct4A expression significantly increases with tumour dedifferentiation. Oct4A expression was also significantly high in the ascites-derived tumour cells of recurrent EOC patients compared to chemonaive patients. Silencing of Oct4A in HEY cells resulted in decreased cellular proliferation, migration, spheroid formation and increased chemosensitivity to cisplatin in vitro. IP injection of Oct4A knockdown cells in vivo produced significantly reduced tumour burden, tumour size and invasiveness in mice, which overall resulted in significantly increased mouse survival rates compared to mice injected with control cells.
Conclusions: This data highlights a crucial role for Oct4A in the progression and metastasis of EOC. Targeting Oct4A may prove to be an effective strategy in the treatment and management of epithelial ovarian tumours.
Figures




References
-
- Coleman MP, Forman D, Bryant H, Butler J, Rachet B, Maringe C. Cancer survival in Australia, Canada, Denmark, Norway, Sweden, and the UK, 1995–2007 (the International Cancer Benchmarking Partnership): an analysis of population-based cancer registry data. Lancet. 2011;377(9760):127–138. doi: 10.1016/S0140-6736(10)62231-3. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

