Increased Expression of DUOX2 Is an Epithelial Response to Mucosal Dysbiosis Required for Immune Homeostasis in Mouse Intestine
- PMID: 26261005
- PMCID: PMC4663159
- DOI: 10.1053/j.gastro.2015.07.062
Increased Expression of DUOX2 Is an Epithelial Response to Mucosal Dysbiosis Required for Immune Homeostasis in Mouse Intestine
Erratum in
-
Correction.Gastroenterology. 2023 May;164(6):1033. doi: 10.1053/j.gastro.2023.02.020. Epub 2023 Mar 21. Gastroenterology. 2023. PMID: 36959022 No abstract available.
Abstract
Background & aims: Dual oxidase 2 (DUOX2), a hydrogen-peroxide generator at the apical membrane of gastrointestinal epithelia, is up-regulated in patients with inflammatory bowel disease (IBD) before the onset of inflammation, but little is known about its effects. We investigated the role of DUOX2 in maintaining mucosal immune homeostasis in mice.
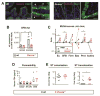
Methods: We analyzed the regulation of DUOX2 in intestinal tissues of germ-free vs conventional mice, mice given antibiotics or colonized with only segmented filamentous bacteria, mice associated with human microbiota, and mice with deficiencies in interleukin (IL) 23 and IL22 signaling. We performed 16S ribosomal RNA gene quantitative polymerase chain reaction of intestinal mucosa and mesenteric lymph nodes of Duoxa(-/-) mice that lack functional DUOX enzymes. Genes differentially expressed in Duoxa(-/-) mice compared with co-housed wild-type littermates were correlated with gene expression changes in early-stage IBD using gene set enrichment analysis.
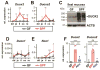
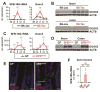
Results: Colonization of mice with segmented filamentous bacteria up-regulated intestinal expression of DUOX2. DUOX2 regulated redox signaling within mucosa-associated microbes and restricted bacterial access to lymphatic tissues of the mice, thereby reducing microbiota-induced immune responses. Induction of Duox2 transcription by microbial colonization did not require the mucosal cytokines IL17 or IL22, although IL22 increased expression of Duox2. Dysbiotic, but not healthy human microbiota, activated a DUOX2 response in recipient germ-free mice that corresponded to abnormal colonization of the mucosa with distinct populations of microbes. In Duoxa(-/-) mice, abnormalities in ileal mucosal gene expression at homeostasis recapitulated those in patients with mucosal dysbiosis.
Conclusions: DUOX2 regulates interactions between the intestinal microbiota and the mucosa to maintain immune homeostasis in mice. Mucosal dysbiosis leads to increased expression of DUOX2, which might be a marker of perturbed mucosal homeostasis in patients with early-stage IBD.
Keywords: Gastroenterology; Inflammatory Bowel Disease; Intestine; Microbial Dysbiosis.
Copyright © 2015 AGA Institute. Published by Elsevier Inc. All rights reserved.
Figures



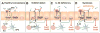
References
-
- Geiszt M, Witta J, Baffi J, et al. Dual oxidases represent novel hydrogen peroxide sources supporting mucosal surface host defense. FASEB J. 2003;17:1502–4. - PubMed
-
- El Hassani RA, Benfares N, Caillou B, et al. Dual oxidase2 is expressed all along the digestive tract. American journal of physiology Gastrointestinal and liver physiology. 2005;288:G933–42. - PubMed
-
- Ha EM, Oh CT, Bae YS, et al. A direct role for dual oxidase in Drosophila gut immunity. Science. 2005;310:847–50. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

