Prostaglandin E2 Promotes Colorectal Cancer Stem Cell Expansion and Metastasis in Mice
- PMID: 26261008
- PMCID: PMC4762503
- DOI: 10.1053/j.gastro.2015.07.064
Prostaglandin E2 Promotes Colorectal Cancer Stem Cell Expansion and Metastasis in Mice
Abstract
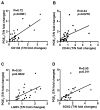
Background & aims: Inflammation may contribute to the formation, maintenance, and expansion of cancer stem cells (CSCs), which have the capacity for self-renewal, differentiation, and resistance to cytotoxic agents. We investigated the effects of the inflammatory mediator prostaglandin E2 (PGE2) on colorectal CSC development and metastasis in mice and the correlation between levels of PGE2 and CSC markers in human colorectal cancer (CRC) specimens.
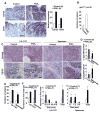
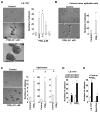
Methods: Colorectal carcinoma specimens and matched normal tissues were collected from patients at the Mayo Clinic (Scottsdale, AZ) and analyzed by mass spectrometry and quantitative polymerase chain reaction. Human primary CRC cells and mouse tumor cells were isolated using microbeads or flow cytometry and analyzed for sphere-formation and by flow cytometry assays. LS-174T cells were sorted by flow cytometry (for CD133(+)CD44(+) and CD133(-)CD44(-) cells) and also used in these assays. NOD-scidIL-2Rγ(-/-) (NSG) mice were given cecal or subcutaneous injections of LS-174T or human primary CRC cells. Apc(Min/+) mice and NSG mice with orthotopic cecal tumors were given vehicle (controls), PGE2, celecoxib, and/or Ono-AE3-208. PGE2 downstream signaling pathways were knocked down with small hairpin RNAs, expressed from lentiviral vectors in LS-174T cells, or blocked with inhibitors in human primary CRC cells.
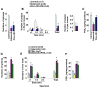
Results: Levels of PGE2 correlated with colonic CSC markers (CD133, CD44, LRG5, and SOX2 messenger RNAs) in human colorectal carcinoma samples. Administration of PGE2 to Apc(Min/+) mice increased tumor stem cells and tumor burden, compared with controls. NSG mice given PGE2 had increased numbers of cecal CSCs and liver metastases compared with controls after intracecal injection of LS-174T or human primary CRC cells. Alternatively, celecoxib, an inhibitor of prostaglandin-endoperoxide synthase 2, reduced polyp numbers in Apc(Min/+) mice, liver metastasis in NSG mice with orthotopic tumors, and numbers of CSCs in Apc(Min/+) and NSG mice. Inhibitors or knockdown of PGE2 receptor 4 (EP4), phosphoinositide 3-kinase (PI3K) p85α, extracellular signal-regulated kinase 1 (ERK1), or nuclear factor (NF)-κB reduced PGE2-induced sphere formation and expansion of LS-174T and/or human primary CRC cells. Knockdown of ERK1 or PI3K p85α also attenuated PGE2-induced activation of NF-κB in LS-174T cells. An EP4 antagonist reduced the ability of PGE2 to induce CSC expansion in orthotopic tumors and to accelerate the formation of liver metastases. Knockdown experiments showed that NF-κB was required for PGE2 induction of CSCs and metastasis in mice.
Conclusions: PGE2 induces CSC expansion by activating NF-κB, via EP4-PI3K and EP4-mitogen-activated protein kinase signaling, and promotes the formation of liver metastases in mice. The PGE2 signaling pathway therefore might be targeted therapeutically to slow CSC expansion and colorectal cancer progression.
Keywords: Colon Cancer; Cyclooxygenase; NSAIDs; Tumorigenesis.
Copyright © 2015 AGA Institute. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest statement: All authors have no any conflict interests
Figures

Similar articles
-
PGE2 /EP4 antagonism enhances tumor chemosensitivity by inducing extracellular vesicle-mediated clearance of cancer stem cells.Int J Cancer. 2018 Sep 15;143(6):1440-1455. doi: 10.1002/ijc.31523. Epub 2018 May 2. Int J Cancer. 2018. PMID: 29658109
-
Prostaglandin E2 Activates YAP and a Positive-Signaling Loop to Promote Colon Regeneration After Colitis but Also Carcinogenesis in Mice.Gastroenterology. 2017 Feb;152(3):616-630. doi: 10.1053/j.gastro.2016.11.005. Epub 2016 Nov 15. Gastroenterology. 2017. PMID: 27864128 Free PMC article.
-
TRIB3 Interacts With β-Catenin and TCF4 to Increase Stem Cell Features of Colorectal Cancer Stem Cells and Tumorigenesis.Gastroenterology. 2019 Feb;156(3):708-721.e15. doi: 10.1053/j.gastro.2018.10.031. Epub 2018 Oct 24. Gastroenterology. 2019. PMID: 30365932
-
Signal transduction cross-talk during colorectal tumorigenesis.Adv Anat Pathol. 2006 Sep;13(5):270-4. doi: 10.1097/01.pap.0000213046.61941.5c. Adv Anat Pathol. 2006. PMID: 16998321 Review.
-
Prostaglandin E2/EP Signaling in the Tumor Microenvironment of Colorectal Cancer.Int J Mol Sci. 2019 Dec 11;20(24):6254. doi: 10.3390/ijms20246254. Int J Mol Sci. 2019. PMID: 31835815 Free PMC article. Review.
Cited by
-
Cancer stem cells induced by chronic stimulation with prostaglandin E2 exhibited constitutively activated PI3K axis.Sci Rep. 2022 Sep 17;12(1):15628. doi: 10.1038/s41598-022-19265-7. Sci Rep. 2022. PMID: 36115905 Free PMC article.
-
Inhibition of skin carcinogenesis by suppression of NF-κB dependent ITGAV and TIMP-1 expression in IL-32γ overexpressed condition.J Exp Clin Cancer Res. 2018 Nov 28;37(1):293. doi: 10.1186/s13046-018-0943-8. J Exp Clin Cancer Res. 2018. PMID: 30486830 Free PMC article.
-
Inhibition of secretory phospholipase A2 IIa attenuates prostaglandin E2-induced invasiveness in lung adenocarcinoma.Mol Cell Biochem. 2019 Jun;456(1-2):145-156. doi: 10.1007/s11010-019-03500-3. Epub 2019 Jan 25. Mol Cell Biochem. 2019. PMID: 30684134
-
The Role of Inflammatory Mediators in Colorectal Cancer Hepatic Metastasis.Cells. 2022 Jul 27;11(15):2313. doi: 10.3390/cells11152313. Cells. 2022. PMID: 35954156 Free PMC article. Review.
-
New frontiers in the treatment of colorectal cancer: Autophagy and the unfolded protein response as promising targets.Autophagy. 2017 May 4;13(5):781-819. doi: 10.1080/15548627.2017.1290751. Epub 2017 Feb 23. Autophagy. 2017. PMID: 28358273 Free PMC article. Review.
References
-
- Newcomb PA, Baron J, Cotterchio M, et al. Colon Cancer Family Registry: an international resource for studies of the genetic epidemiology of colon cancer. Cancer Epidemiol Biomarkers Prev. 2007;16:2331–43. - PubMed
-
- de Groot DJ, de Vries EG, Groen HJ, et al. Non-steroidal anti-inflammatory drugs to potentiate chemotherapy effects: from lab to clinic. Crit Rev Oncol Hematol. 2007;61:52–69. - PubMed
-
- Cuzick J, Otto F, Baron JA, et al. Aspirin and non-steroidal anti-inflammatory drugs for cancer prevention: an international consensus statement. Lancet Oncol. 2009;10:501–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

