Attitudes of clinicians following large-scale pharmacogenomics implementation
- PMID: 26261062
- PMCID: PMC4751074
- DOI: 10.1038/tpj.2015.57
Attitudes of clinicians following large-scale pharmacogenomics implementation
Abstract
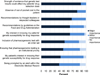
Clinician attitudes toward multiplexed genomic testing may be vital to the success of translational programs. We surveyed clinicians at an academic medical center about their views on a large pharmacogenomics implementation, the PREDICT (Pharmacogenomic Resource for Enhanced Decisions in Care and Treatment) program. Participants were asked about test ordering, major factors influencing use of results, expectations of efficacy and responsibility for applying results to patient care. Virtually all respondents (99%) agreed that pharmacogenomics variants influence patients' response to drug therapy. The majority (92%) favored immediate, active notification when a clinically significant drug-genome interaction was present. However, clinicians were divided on which providers were responsible for acting on a result when a prescription change was indicated and whether patients should be directly notified of a significant result. We concluded genotype results were valued for tailoring prescriptions, but clinicians do not agree on how to appropriately assign clinical responsibility for actionable results from a multiplexed panel.The Pharmacogenomics Journal advance online publication, 11 August 2015; doi:10.1038/tpj.2015.57.
Conflict of interest statement
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

