A stress-responsive NAC transcription factor SNAC3 confers heat and drought tolerance through modulation of reactive oxygen species in rice
- PMID: 26261267
- PMCID: PMC4623689
- DOI: 10.1093/jxb/erv386
A stress-responsive NAC transcription factor SNAC3 confers heat and drought tolerance through modulation of reactive oxygen species in rice
Abstract
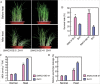
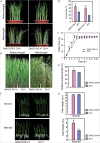
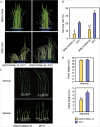
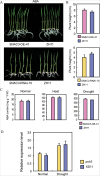
Adverse environmental conditions such as high temperature and drought stress greatly limit the growth and production of crops worldwide. Several NAC (NAM, ATAF1/2, and CUC2) proteins have been documented as important regulators in stress responses, but the molecular mechanisms are largely unknown. Here, a stress-responsive NAC gene, SNAC3 (ONAC003, LOC_Os01g09550), conferring drought and heat tolerance in rice is reported. SNAC3 was ubiquitously expressed and its transcript level was induced by drought, high temperature, salinity stress, and abscisic acid (ABA) treatment. Overexpression (OE) of SNAC3 in rice resulted in enhanced tolerance to high temperature, drought, and oxidative stress caused by methyl viologen (MV), whereas suppression of SNAC3 by RNAi resulted in increased sensitivity to these stresses. The SNAC3-OE transgenic plants exhibited significantly lower levels of H2O2, malondiadehyde (MDA), and relative electrolyte leakage than the wild-type control under heat stress conditions, implying that SNAC3 may confer stress tolerance by modulating reactive oxygen species (ROS) homeostasis. Quantitative PCR experiments showed that the expression of a large number of ROS-scavenging genes was dramatically increased in the SNAC3-OE plants, but significantly decreased in the SNAC3-RNAi transgenic plants. Five ROS-associated genes which were up-regulated in SNAC3-OE plants showed co-expression patterns with SNAC3, and three of the co-expressed ROS-associated enzyme genes were verified to be direct target genes of SNAC3. These results suggest that SNAC3 plays important roles in stress responses, and it is likely to be useful for engineering crops with improved tolerance to heat and drought stress.
Keywords: Abiotic stress; NAC; Oryza sativa; ROS; transcription factors..
© The Author 2015. Published by Oxford University Press on behalf of the Society for Experimental Biology.
Figures




References
-
- Agarie S, Hanaoka N, Ueno O, Miyazaki A, Kubota F, Agata W, Kaufman PB. 1998. Effects of silicon on tolerance to water deficit and heat stress in rice plants (Oryza sativa L.), monitored by electrolyte leakage. Plant Production Science 1, 96–103.
-
- Apel K, Hirt H. 2004. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annual Review of Plant Biology 55, 373–399. - PubMed
-
- Bajji M, Kinet J M, Lutts S. 2002. The use of the electrolyte leakage method for assessing cell membrane stability as a water stress tolerance test in durum wheat. Plant Growth Regulation 36, 61–70.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

