Mechanical Forces and Growth in Animal Tissues
- PMID: 26261279
- PMCID: PMC4772101
- DOI: 10.1101/cshperspect.a019232
Mechanical Forces and Growth in Animal Tissues
Abstract
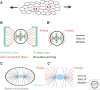
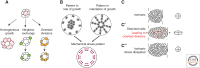
Mechanical forces shape biological tissues. They are the effectors of the developmental programs that orchestrate morphogenesis. A lot of effort has been devoted to understanding morphogenetic processes in mechanical terms. In this review, we focus on the interplay between tissue mechanics and growth. We first describe how tissue mechanics affects growth, by influencing the orientation of cell divisions and the signaling pathways that control the rate of volume increase and proliferation. We then address how the mechanical state of a tissue is affected by the patterns of growth. The forward and reverse interactions between growth and mechanics must be investigated in an integrative way if we want to understand how tissues grow and shape themselves. To illustrate this point, we describe examples in which growth homeostasis is achieved by feedback mechanisms that use mechanical forces.
Copyright © 2016 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures


References
-
- Aegerter-Wilmsen T, Aegerter CM, Hafen E, Basler K. 2007. Model for the regulation of size in the wing imaginal disc of Drosophila. Mech Dev 124: 318–326. - PubMed
-
- Aigouy B, Farhadifar R, Staple DB, Sagner A, Röper J-C, Jülicher F, Eaton S. 2010. Cell flow reorients the axis of planar polarity in the wing epithelium of Drosophila. Cell 142: 773–786. - PubMed
-
- Aragona M, Panciera T, Manfrin A, Giulitti S, Michielin F, Elvassore N, Dupont S, Piccolo S. 2013. A mechanical checkpoint controls multicellular growth through YAP/TAZ regulation by actin-processing factors. Cell 154: 1047–1059. - PubMed
-
- Baena-Lopez LA, Baonza A, Garcia-Bellido A. 2005. The orientation of cell divisions determines the shape of Drosophila organs. Curr Biol 15: 1640–1644. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
