The Systemic Control of Growth
- PMID: 26261282
- PMCID: PMC4665074
- DOI: 10.1101/cshperspect.a019117
The Systemic Control of Growth
Abstract
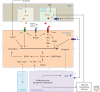
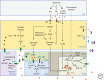
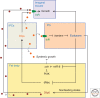
Growth is a complex process that is intimately linked to the developmental program to form adults with proper size and proportions. Genetics is an important determinant of growth, as exemplified by the role of local diffusible molecules setting up organ proportions. In addition, organisms use adaptive responses allowing modulating the size of individuals according to environmental cues, for example, nutrition. Here, we describe some of the physiological principles participating in the determination of final individual size.
Copyright © 2015 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures
References
-
- Adeghate E, Ponery AS, Wahab A. 2001. Effect of electrical field stimulation on insulin and glucagon secretion from the pancreas of normal and diabetic rats. Horm Metab Res 33: 281–289. - PubMed
-
- Andersen DS, Colombani J, Léopold P. 2013. Coordination of organ growth: Principles and outstanding questions from the world of insects. Trends Cell Biol 23: 336–344. - PubMed
-
- Arquier N, Géminard C, Bourouis M, Jarretou G, Honegger B, Paix A, Léopold P. 2008. Drosophila ALS regulates growth and metabolism through functional interaction with insulin-like peptides. Cell Metab 7: 333–338. - PubMed
-
- Arsham AM, Neufeld TP. 2006. Thinking globally and acting locally with TOR. Curr Opin Cell Biol 18: 589–597. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
