Antibodies targeting human IL1RAP (IL1R3) show therapeutic effects in xenograft models of acute myeloid leukemia
- PMID: 26261316
- PMCID: PMC4553807
- DOI: 10.1073/pnas.1422749112
Antibodies targeting human IL1RAP (IL1R3) show therapeutic effects in xenograft models of acute myeloid leukemia
Abstract
Acute myeloid leukemia (AML) is associated with a poor survival rate, and there is an urgent need for novel and more efficient therapies, ideally targeting AML stem cells that are essential for maintaining the disease. The interleukin 1 receptor accessory protein (IL1RAP; IL1R3) is expressed on candidate leukemic stem cells in the majority of AML patients, but not on normal hematopoietic stem cells. We show here that monoclonal antibodies targeting IL1RAP have strong antileukemic effects in xenograft models of human AML. We demonstrate that effector-cell-mediated killing is essential for the observed therapeutic effects and that natural killer cells constitute a critical human effector cell type. Because IL-1 signaling is important for the growth of AML cells, we generated an IL1RAP-targeting antibody capable of blocking IL-1 signaling and show that this antibody suppresses the proliferation of primary human AML cells. Hence, IL1RAP can be efficiently targeted with an anti-IL1RAP antibody capable of both achieving antibody-dependent cellular cytotoxicity and blocking of IL-1 signaling as modes of action. Collectively, these results provide important evidence in support of IL1RAP as a target for antibody-based treatment of AML.
Keywords: AML; IL1RAP; antibody; immunotherapy; leukemia.
Conflict of interest statement
Conflict of interest statement: K.S., M.J., and T.F. are cofounders of Cantargia AB (Medicon Village, Lund), formed together with Lund University Bioscience AB. Cantargia AB is the owner of the intellectual property rights for agents targeting IL1RAP for use in the treatment and diagnosis of neoplastic hematologic disorders. J.R. has stock options in Cantargia.
Figures

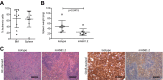



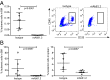


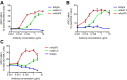


References
-
- Burnett A, Wetzler M, Löwenberg B. Therapeutic advances in acute myeloid leukemia. J Clin Oncol. 2011;29(5):487–494. - PubMed
-
- Hope KJ, Jin L, Dick JE. Acute myeloid leukemia originates from a hierarchy of leukemic stem cell classes that differ in self-renewal capacity. Nat Immunol. 2004;5(7):738–743. - PubMed
-
- Ishikawa F, et al. Chemotherapy-resistant human AML stem cells home to and engraft within the bone-marrow endosteal region. Nat Biotechnol. 2007;25(11):1315–1321. - PubMed
-
- Hoelzer D. Targeted therapy with monoclonal antibodies in acute lymphoblastic leukemia. Curr Opin Oncol. 2013;25(6):701–706. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

