The amino acid sensor GCN2 inhibits inflammatory responses to apoptotic cells promoting tolerance and suppressing systemic autoimmunity
- PMID: 26261340
- PMCID: PMC4553766
- DOI: 10.1073/pnas.1504276112
The amino acid sensor GCN2 inhibits inflammatory responses to apoptotic cells promoting tolerance and suppressing systemic autoimmunity
Abstract
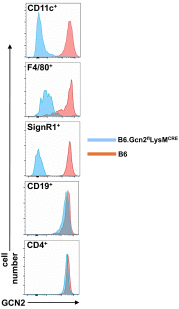
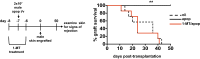
Efficient apoptotic cell clearance and induction of immunologic tolerance is a critical mechanism preventing autoimmunity and associated pathology. Our laboratory has reported that apoptotic cells induce tolerance by a mechanism dependent on the tryptophan catabolizing enzyme indoleamine 2,3 dioxygenase 1 (IDO1) in splenic macrophages (MΦ). The metabolic-stress sensing protein kinase GCN2 is a primary downstream effector of IDO1; thus, we tested its role in apoptotic cell-driven immune suppression. In vitro, expression of IDO1 in MΦs significantly enhanced apoptotic cell-driven IL-10 and suppressed IL-12 production in a GCN2-dependent mechanism. Suppression of IL-12 protein production was due to attenuation of IL-12 mRNA association with polyribosomes inhibiting translation while IL-10 mRNA association with polyribosomes was not affected. In vivo, apoptotic cell challenge drove a rapid, GCN2-dependent stress response in splenic MΦs with increased IL-10 and TGF-β production, whereas myeloid-specific deletion of GCN2 abrogated regulatory cytokine production with provocation of inflammatory T-cell responses to apoptotic cell antigens and failure of long-tolerance induction. Consistent with a role in prevention of apoptotic cell driven autoreactivity, myeloid deletion of GCN2 in lupus-prone mice resulted in increased immune cell activation, humoral autoimmunity, renal pathology, and mortality. In contrast, activation of GCN2 with an agonist significantly reduced anti-DNA autoantibodies and protected mice from disease. Thus, this study implicates a key role for GCN2 signals in regulating the tolerogenic response to apoptotic cells and limiting autoimmunity.
Keywords: apoptosis; autoimmunity; immunometabolism; stress; tolerance.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Rosen A, Casciola-Rosen L. Autoantigens as substrates for apoptotic proteases: Implications for the pathogenesis of systemic autoimmune disease. Cell Death Differ. 1999;6(1):6–12. - PubMed
-
- McGaha TL, Chen Y, Ravishankar B, van Rooijen N, Karlsson MC. Marginal zone macrophages suppress innate and adaptive immunity to apoptotic cells in the spleen. Blood. 2011;117(20):5403–5412. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

