The opportunistic marine pathogen Vibrio parahaemolyticus becomes virulent by acquiring a plasmid that expresses a deadly toxin
- PMID: 26261348
- PMCID: PMC4553777
- DOI: 10.1073/pnas.1503129112
The opportunistic marine pathogen Vibrio parahaemolyticus becomes virulent by acquiring a plasmid that expresses a deadly toxin
Erratum in
-
Correction for Lee et al., The opportunistic marine pathogen Vibrio parahaemolyticus becomes virulent by acquiring a plasmid that expresses a deadly toxin.Proc Natl Acad Sci U S A. 2015 Sep 29;112(39):E5445. doi: 10.1073/pnas.1517100112. Epub 2015 Sep 4. Proc Natl Acad Sci U S A. 2015. PMID: 26340988 Free PMC article. No abstract available.
Abstract
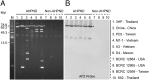
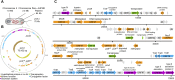
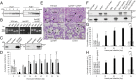
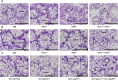
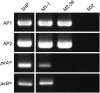
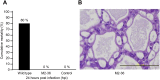
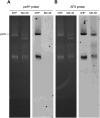
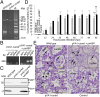
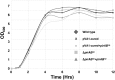
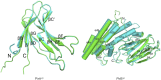
Acute hepatopancreatic necrosis disease (AHPND) is a severe, newly emergent penaeid shrimp disease caused by Vibrio parahaemolyticus that has already led to tremendous losses in the cultured shrimp industry. Until now, its disease-causing mechanism has remained unclear. Here we show that an AHPND-causing strain of V. parahaemolyticus contains a 70-kbp plasmid (pVA1) with a postsegregational killing system, and that the ability to cause disease is abolished by the natural absence or experimental deletion of the plasmid-encoded homologs of the Photorhabdus insect-related (Pir) toxins PirA and PirB. We determined the crystal structure of the V. parahaemolyticus PirA and PirB (PirA(vp) and PirB(vp)) proteins and found that the overall structural topology of PirA(vp)/PirB(vp) is very similar to that of the Bacillus Cry insecticidal toxin-like proteins, despite the low sequence identity (<10%). This structural similarity suggests that the putative PirAB(vp) heterodimer might emulate the functional domains of the Cry protein, and in particular its pore-forming activity. The gene organization of pVA1 further suggested that pirAB(vp) may be lost or acquired by horizontal gene transfer via transposition or homologous recombination.
Keywords: AHPND; Pir toxin; Vibrio parahaemolyticus; shrimp; virulence plasmid.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- NACA-FAO . Quarterly Aquatic Animal Disease Report (Asia and Pacific Region), 2011/2, April-June 2011. NACA; Bangkok: 2011.
-
- Lightner DV, Redman RM, Pantoja CR, Noble BL, Tran L. Early mortality syndrome affects shrimp in Asia. Global Aquaculture Advocate. 2012;15:40.
-
- Nunan L, Lightner D, Pantoja C, Gomez-Jimenez S. Detection of acute hepatopancreatic necrosis disease (AHPND) in Mexico. Dis Aquat Organ. 2014;111(1):81–86. - PubMed
-
- FAO Fisheries and Aquaculture Report of the FAO/MARD Technical Workshop on Early Mortality Syndrome (EMS) or Acute Hepatopancreatic Necrosis Syndrome (AHPNS) of Cultured Shrimp (under TCP/VIE/3304) 2013 rep. no. 1053, Hanoi, Viet Nam, 25–27 June 2013. Available at www.fao.org/docrep/018/i3422e/i3422e.pdf. Accessed July 28, 2015.
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

