Imaging preoperatively for pancreatic adenocarcinoma
- PMID: 26261722
- PMCID: PMC4502157
- DOI: 10.3978/j.issn.2078-6891.2015.024
Imaging preoperatively for pancreatic adenocarcinoma
Abstract
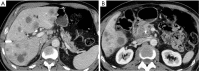
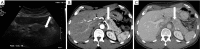
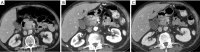
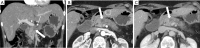
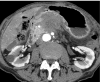
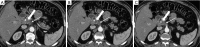
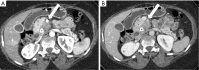
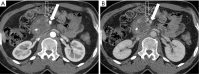
Pancreatic cancer is a highly lethal malignancy which is increasing in incidence and mortality. The fourth leading cause of cancer death in the U.S., pancreatic cancer is projected to become the second leading cause of cancer death by 2020. Patients with pancreatic cancer have an abysmal 5-year survival of 6%, and 90% of these patients eventually die from the disease. This is in large part due to the commonly advanced stage of disease at the time of diagnosis. Currently, the only potentially curative therapy for pancreatic carcinoma is complete surgical resection. Patients who undergo incomplete resection with residual disease have similar survival rates to those patients with metastatic disease and should be spared this relatively morbid surgery. Thus, the key to impacting prognosis is the detection of smaller and earlier stage lesions, and the key to optimal management is accurately determining which patients have potentially resectable surgery and which patients would not benefit from surgery. Cross-sectional imaging plays an essential role in both the diagnosis and appropriate staging of pancreatic carcinoma. The diagnosis and staging of pancreatic adenocarcinoma is performed with cross-sectional imaging. Multi-detector computed tomography (MDCT) is the most commonly used, best-validated imaging modality for the diagnosis and staging of pancreatic cancer. Modern contrast-enhanced magnetic resonance imaging (MRI) has been demonstrated to be equivalent to MDCT in detection and staging of pancreatic cancer. Endoscopic ultrasound (EUS) is very sensitive for detecting pancreatic masses; however, due to limitations in adequate overall abdominal staging, it is generally used in addition to or after MDCT. Transabdominal ultrasound and positron emission tomography/computed tomography (PET/CT) have limited roles in the diagnosis and staging of pancreatic cancer. Preoperative imaging is used to characterize patients as having resectable disease, borderline resectable disease, locally advanced disease (unresectable) and metastatic disease (unresectable). As the definitions of borderline resectable and unresectable may vary from institution to institution and within institutions, it is essential to accurately assess and describe the factors relevant to staging including: local extent of tumor, vascular involvement, lymph node involvement and distant metastatic disease. To facilitate this, standardized reporting templates for pancreatic ductal adenocarcinoma have been created and published. Structured reporting for pancreatic cancer has been reported to provide superior evaluation of pancreatic cancer, facilitate surgical planning, and increase surgeons' confidence about tumor resectability.
Keywords: Pancreatic cancer; magnetic resonance imaging (MRI); multi-detector computed tomography (MDCT); staging.
Figures
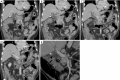
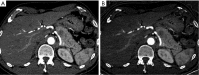
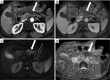
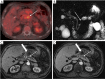

References
-
- Matrisian LM, Aizenberg R, Rosenzweig A. The alarming rise of pancreatic cancer deaths in the United States: why we need to stem the tide today. Available online: https://www.pancan.org/wp-content/uploads/2013/01/incidence_report_2012.pdf
-
- Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin 2014;64:9-29. - PubMed
-
- Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med 2014;371:1039-49. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
