Ex Vivo Perfusion With Adenosine A2A Receptor Agonist Enhances Rehabilitation of Murine Donor Lungs After Circulatory Death
- PMID: 26262504
- PMCID: PMC4668207
- DOI: 10.1097/TP.0000000000000830
Ex Vivo Perfusion With Adenosine A2A Receptor Agonist Enhances Rehabilitation of Murine Donor Lungs After Circulatory Death
Abstract
Background: Ex vivo lung perfusion (EVLP) enables assessment and rehabilitation of marginal donor lungs before transplantation. We previously demonstrated that adenosine A2A receptor (A2AR) agonism attenuates lung ischemia-reperfusion injury. The current study utilizes a novel murine EVLP model to test the hypothesis that A2AR agonist enhances EVLP-mediated rehabilitation of donation after circulatory death (DCD) lungs.
Methods: Mice underwent euthanasia and 60 minutes warm ischemia, and lungs were flushed with Perfadex and underwent cold static preservation (CSP, 60 minutes). Three groups were studied: no EVLP (CSP), EVLP with Steen solution for 60 minutes (EVLP), and EVLP with Steen solution supplemented with ATL1223, a selective A2AR agonist (EVLP + ATL1223). Lung function, wet/dry weight, cytokines and neutrophil numbers were measured. Microarrays were performed using the Affymetrix GeneChip Mouse Genome 430A 2.0 Array.
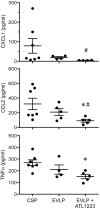
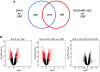
Results: Ex vivo lung perfusion significantly improved lung function versus CSP, which was further, significantly improved by EVLP + ATL1223. Lung edema, cytokines, and neutrophil counts were reduced after EVLP and further, significantly reduced after EVLP + ATL1223. Gene array analysis revealed differential expression of 1594 genes after EVLP, which comprise canonical pathways involved in inflammation and innate immunity including IL-1, IL-8, IL-6, and IL-17 signaling. Several pathways were uniquely regulated by EVLP + ATL1223 including the downregulation of genes involved in IL-1 signaling, such as ADCY9, ECSIT, IRAK1, MAPK12, and TOLLIP.
Conclusions: Ex vivo lung perfusion modulates proinflammatory genes and reduces pulmonary dysfunction, edema, and inflammation in DCD lungs, which are further reduced by A2AR agonism. This murine EVLP model provides a novel platform to study rehabilitative mechanisms of DCD lungs.
Conflict of interest statement
Figures




References
-
- Cypel M, Rubacha M, Yeung J, et al. Normothermic ex vivo perfusion prevents lung injury compared to extended cold preservation for transplantation. Am J Transplant. 2009;9:2262. - PubMed
-
- de Perrot M, Liu M, Waddell TK, Keshavjee S. Ischemia-reperfusion-induced lung injury. Am J Respir Crit Care Med. 2003;167:490. - PubMed
-
- Sanchez PG, Bittle GJ, Williams K, et al. Ex vivo lung evaluation of prearrest heparinization in donation after cardiac death. Ann Surg. 2013;257:534. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

