Comparison of Soybean Transformation Efficiency and Plant Factors Affecting Transformation during the Agrobacterium Infection Process
- PMID: 26262617
- PMCID: PMC4581258
- DOI: 10.3390/ijms160818522
Comparison of Soybean Transformation Efficiency and Plant Factors Affecting Transformation during the Agrobacterium Infection Process
Abstract
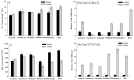
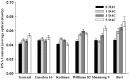
The susceptibility of soybean genotype to Agrobacterium infection is a key factor for the high level of genetic transformation efficiency. The objective of this study is to evaluate the plant factors related to transformation in cotyledonary nodes during the Agrobacterium infection process. This study selected three genotypes (Williams 82, Shennong 9 and Bert) with high transformation efficiency, which presented better susceptibility to Agrobacterium infection, and three low transformation efficiency genotypes (General, Liaodou 16 and Kottman), which showed a relatively weak susceptibility. Gibberellin (GA) levels and soybean GA20ox2 and CYP707A2 transcripts of high-efficiency genotypes increased and were higher than those of low-efficiency genotypes; however, the opposite performance was shown in abscisic acid (ABA). Higher zeatin riboside (ZR) content and DNA quantity, and relatively higher expression of soybean IPT5, CYCD3 and CYCA3 were obtained in high-efficiency genotypes. High-efficiency genotypes had low methyl jasmonate (MeJA) content, polyphenol oxidase (PPO) and peroxidase (POD) activity, and relatively lower expression of soybean OPR3, PPO1 and PRX71. GA and ZR were positive plant factors for Agrobacterium-mediated soybean transformation by facilitating germination and growth, and increasing the number of cells in DNA synthesis cycle, respectively; MeJA, PPO, POD and ABA were negative plant factors by inducing defence reactions and repressing germination and growth, respectively.
Keywords: Agrobacterium infection; cotyledonary node; plant factors; soybean; transformation.
Figures


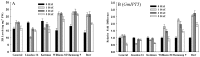



References
-
- James C. The International Service for the Acquisition of Agri-Biotech Applications Brief. The International Service for the Acquisition of Agri-biotech Applications; Ithaca, NY, USA: 2012. Global status of commercialized biotech/GM crops: 2012. No. 44.
-
- Miklos J.A., Alibhai M.F., Bledig S.A., Connor-Ward D.C., Gao A.G., Holmes B.A., Kolacz K.H., Kabuye V.T., MacRae T.C., Paradise M.S., et al. Characterization of soybean exhibiting high expression of a synthetic Bacillus thuringiensis cry1A transgene that confers a high degree of resistance to lepidopteran pests. Crop Sci. Soc. Am. 2007;47:148–157.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

