Prostaglandin E2 from Candida albicans Stimulates the Growth of Staphylococcus aureus in Mixed Biofilms
- PMID: 26262843
- PMCID: PMC4532413
- DOI: 10.1371/journal.pone.0135404
Prostaglandin E2 from Candida albicans Stimulates the Growth of Staphylococcus aureus in Mixed Biofilms
Abstract
Background: Previous studies showed that Staphylococcus aureus and Candida albicans interact synergistically in dual species biofilms resulting in enhanced mortality in animal models.
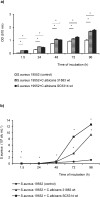
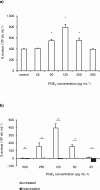
Methodology/principal findings: The aim of the current study was to test possible candidate molecules which might mediate this synergistic interaction in an in vitro model of mixed biofilms, such as farnesol, tyrosol and prostaglandin (PG) E2. In mono-microbial and dual biofilms of C.albicans wild type strains PGE2 levels between 25 and 250 pg/mL were measured. Similar concentrations of purified PGE2 significantly enhanced S.aureus biofilm formation in a mode comparable to that observed in dual species biofilms. Supernatants of the null mutant deficient in PGE2 production did not stimulate the proliferation of S.aureus and the addition of the cyclooxygenase inhibitor indomethacin blocked the S.aureus biofilm formation in a dose-dependent manner. Additionally, S. aureus biofilm formation was boosted by low and inhibited by high farnesol concentrations. Supernatants of the farnesol-deficient C. albicans ATCC10231 strain significantly enhanced the biofilm formation of S. aureus but at a lower level than the farnesol producer SC5314. However, C. albicans ATCC10231 also produced PGE2 but amounts were significantly lower compared to SC5314.
Conclusion/significance: In conclision, we identified C. albicans PGE2 as a key molecule stimulating the growth and biofilm formation of S. aureus in dual S. aureus/C. albicans biofilms, although C. albicans derived farnesol, but not tyrosol, may also contribute to this effect but to a lesser extent.
Conflict of interest statement
Figures




References
-
- Staib F, Grosse G, Mishra SK. Staphylococcus aureus and Candida albicans infection (animal experiments). Zentralbl Bakteriol. 1976; 234: 450–461. - PubMed
-
- deRepentigny J, Levesque R, Mathieu LG. Increase in the in vitro susceptibility of Staphylococcus aureus to antimicrobial agents in the presence of Candida albicans. Can J Microbiol. 1979; 25: 429–435. - PubMed
-
- Hogan DA, Vik A, Kolter R. A Pseudomonas aeruginosa quorum-sensing molecule influences Candida albicans morphology. Mol Microbiol. 2004; 54: 1212–1223. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

