The Role of MKP-1 in the Anti-Proliferative Effects of Glucocorticoids in Primary Rat Pre-Osteoblasts
- PMID: 26263165
- PMCID: PMC4532462
- DOI: 10.1371/journal.pone.0135358
The Role of MKP-1 in the Anti-Proliferative Effects of Glucocorticoids in Primary Rat Pre-Osteoblasts
Abstract
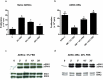
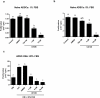
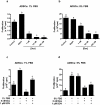
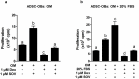
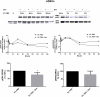
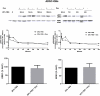
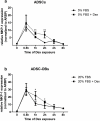
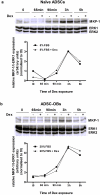
Glucocorticoid (GC)-induced osteoporosis has been attributed to a GC-induced suppression of pre-osteoblast proliferation. Our previous work identified a critical role for mitogen-activated protein kinase (MAPK) phosphatase-1 (MKP-1) in mediating the anti-proliferative effects of GCs in immortalized pre-osteoblasts, but we subsequently found that MKP-1 null mice were not protected against the pathological effects of GCs on bone. In order to reconcile this discrepancy, we have assessed the effects of GCs on proliferation, activation of the MAPK ERK1/2 and MKP-1 expression in primary adipose-derived stromal cells (ADSCs) and ADSC-derived pre-osteoblasts (ADSC-OBs). ADSCs were isolated by means of collagenase digestion from adipose tissue biopsies harvested from adult male Wistar rats. ADSC-OBs were prepared by treating ADSCs with osteoblast differentiation media for 7 days. The effects of increasing concentrations of the GC dexamethasone on basal and mitogen-stimulated cell proliferation were quantified by tritiated thymidine incorporation. ERK1/2 activity was measured by Western blotting, while MKP-1 expression was quantified on both RNA and protein levels, using semi-quantitative real-time PCR and Western blotting, respectively. GCs were strongly anti-proliferative in both naïve ADSCs and ADSC-OBs, but had very little effect on mitogen-induced ERK1/2 activation and did not upregulate MKP-1 protein expression. These findings suggest that the anti-proliferative effects of GCs in primary ADSCs and ADSC-OBs in vitro do not require the inhibition of ERK1/2 activation by MKP-1, which is consistent with our in vivo findings in MKP-1 null mice.
Conflict of interest statement
Figures
Similar articles
-
Mitogen-activated protein kinase phosphatase 1/dual specificity phosphatase 1 mediates glucocorticoid inhibition of osteoblast proliferation.Mol Endocrinol. 2007 Dec;21(12):2929-40. doi: 10.1210/me.2007-0153. Epub 2007 Aug 30. Mol Endocrinol. 2007. PMID: 17761948 Free PMC article.
-
Expression of mitogen-activated protein kinase phosphatase 1, a negative regulator of the mitogen-activated protein kinases, in rheumatoid arthritis: up-regulation by interleukin-1beta and glucocorticoids.Arthritis Rheum. 2004 Oct;50(10):3118-28. doi: 10.1002/art.20580. Arthritis Rheum. 2004. PMID: 15476200
-
MKP-1 mediates glucocorticoid-induced ERK1/2 dephosphorylation and reduction in pancreatic ß-cell proliferation in islets from early lactating mothers.Am J Physiol Endocrinol Metab. 2010 Dec;299(6):E1006-15. doi: 10.1152/ajpendo.00341.2010. Epub 2010 Sep 21. Am J Physiol Endocrinol Metab. 2010. PMID: 20858750
-
Distinct roles for mitogen-activated protein kinase phosphatase-1 (MKP-1) and ERK-MAPK in PTH1R signaling during osteoblast proliferation and differentiation.Cell Signal. 2010 Mar;22(3):457-66. doi: 10.1016/j.cellsig.2009.10.017. Cell Signal. 2010. PMID: 19892016 Free PMC article.
-
MKP-1 knockout does not prevent glucocorticoid-induced bone disease in mice.Calcif Tissue Int. 2011 Sep;89(3):221-7. doi: 10.1007/s00223-011-9509-x. Epub 2011 Jun 23. Calcif Tissue Int. 2011. PMID: 21698455
Cited by
-
Vanadate Impedes Adipogenesis in Mesenchymal Stem Cells Derived from Different Depots within Bone.Front Endocrinol (Lausanne). 2016 Aug 3;7:108. doi: 10.3389/fendo.2016.00108. eCollection 2016. Front Endocrinol (Lausanne). 2016. PMID: 27536268 Free PMC article.
-
Protective effect of VK2 on glucocorticoid-treated MC3T3-E1 cells.Int J Mol Med. 2017 Jan;39(1):160-166. doi: 10.3892/ijmm.2016.2817. Epub 2016 Dec 1. Int J Mol Med. 2017. PMID: 27909721 Free PMC article.
References
-
- Canalis E. Clinical review 83: Mechanisms of glucocorticoid action in bone: implications to glucocorticoid-induced osteoporosis. J Clin Endocrinol Metab. 1996; 81: 3441–3447. - PubMed
-
- Van Staa TP, Leufkens HG, Abenhaim L, Zhang B, Cooper C. Use of oral corticosteroids and risk of fractures. J Bone Miner Res. 2000; 15: 993–1000. - PubMed
-
- Canalis E, Mazziotti G, Giustina A, Bilezikian JP. Glucocorticoid-induced osteoporosis: pathophysiology and therapy. Osteoporos Int. 2007; 18: 1319–1328. - PubMed
-
- Ishida Y, Heersche JN. Glucocorticoid-induced osteoporosis: both in vivo and in vitro concentrations of glucocorticoids higher than physiological levels attenuate osteoblast differentiation. J Bone Miner Res. 1998; 13: 1822–1826. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

