Efficient Generation of Plasmacytoid Dendritic Cell from Common Lymphoid Progenitors by Flt3 Ligand
- PMID: 26263178
- PMCID: PMC4532451
- DOI: 10.1371/journal.pone.0135217
Efficient Generation of Plasmacytoid Dendritic Cell from Common Lymphoid Progenitors by Flt3 Ligand
Abstract
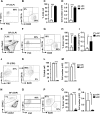
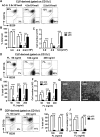
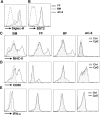
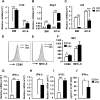
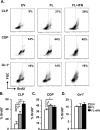
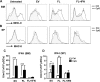
Dendritic cells (DCs), including conventional DCs (cDCs) and plasmacytoid DCs (pDCs) are critical for initiating and controlling the immune response. However, study of DC, particularly pDC, function is hampered by their low frequency in lymphoid organs, and existing methods for in vitro DC generation preferentially favor the production of cDCs over pDCs. Here, we demonstrated that pDCs could be efficiently generated in vitro from common lymphoid progenitors (CLPs) using Flt3 ligand (FL) in three different culture systems, namely feeder-free, BM-feeder and AC-6-feeder. This was in stark contrast to common DC progenitors (CDPs), in which cDCs were prominently generated under the same conditions. Moreover, the efficiency and function of pDCs generated from these three systems varied. While AC-6 system showed the greatest ability to support pDC development from CLPs, BM-feeder system was able to develop pDCs with better functionality. pDCs could also be expanded in vivo using hydrodynamic gene transfer of FL, which was further enhanced by the combined treatment of FL and IFN-α. Interestingly, IFN-α selectively promoted the proliferation of CLPs and not CDPs, which might contribute to enhanced pDC development. Together, we have defined conditions for in vitro and in vivo generation of pDCs, which may be useful for investigating the biology of pDCs.
Conflict of interest statement
Figures

Similar articles
-
In Vitro Generation of Murine Plasmacytoid Dendritic Cells from Common Lymphoid Progenitors using the AC-6 Feeder System.J Vis Exp. 2015 Nov 23;(105):53211. doi: 10.3791/53211. J Vis Exp. 2015. PMID: 26650046 Free PMC article.
-
A type I IFN-Flt3 ligand axis augments plasmacytoid dendritic cell development from common lymphoid progenitors.J Exp Med. 2013 Nov 18;210(12):2515-22. doi: 10.1084/jem.20130536. Epub 2013 Oct 21. J Exp Med. 2013. PMID: 24145513 Free PMC article.
-
TGF-beta1 accelerates dendritic cell differentiation from common dendritic cell progenitors and directs subset specification toward conventional dendritic cells.J Immunol. 2010 Nov 1;185(9):5326-35. doi: 10.4049/jimmunol.0903950. Epub 2010 Sep 29. J Immunol. 2010. PMID: 20881193
-
What Makes a pDC: Recent Advances in Understanding Plasmacytoid DC Development and Heterogeneity.Front Immunol. 2019 May 29;10:1222. doi: 10.3389/fimmu.2019.01222. eCollection 2019. Front Immunol. 2019. PMID: 31191558 Free PMC article. Review.
-
Development of dendritic cell system.Cell Mol Immunol. 2004 Apr;1(2):112-8. Cell Mol Immunol. 2004. PMID: 16212897 Review.
Cited by
-
In Vitro Generation of Murine Plasmacytoid Dendritic Cells from Common Lymphoid Progenitors using the AC-6 Feeder System.J Vis Exp. 2015 Nov 23;(105):53211. doi: 10.3791/53211. J Vis Exp. 2015. PMID: 26650046 Free PMC article.
-
Flt3L Treatment of Bone Marrow Donors Increases Graft Plasmacytoid Dendritic Cell Content and Improves Allogeneic Transplantation Outcomes.Biol Blood Marrow Transplant. 2019 Jun;25(6):1075-1084. doi: 10.1016/j.bbmt.2018.11.029. Epub 2018 Nov 29. Biol Blood Marrow Transplant. 2019. PMID: 30503387 Free PMC article.
-
Immunomodulatory Arming Factors-The Current Paradigm for Oncolytic Vectors Relies on Immune Stimulating Molecules.Int J Mol Sci. 2021 Aug 22;22(16):9051. doi: 10.3390/ijms22169051. Int J Mol Sci. 2021. PMID: 34445759 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

