The Novel Cyclophilin Inhibitor CPI-431-32 Concurrently Blocks HCV and HIV-1 Infections via a Similar Mechanism of Action
- PMID: 26263487
- PMCID: PMC4532424
- DOI: 10.1371/journal.pone.0134707
The Novel Cyclophilin Inhibitor CPI-431-32 Concurrently Blocks HCV and HIV-1 Infections via a Similar Mechanism of Action
Abstract
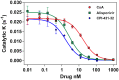
HCV-related liver disease is the main cause of morbidity and mortality of HCV/HIV-1 co-infected patients. Despite the recent advent of anti-HCV direct acting antivirals (DAAs), the treatment of HCV/HIV-1 co-infected patients remains a challenge, as these patients are refractory to most therapies and develop liver fibrosis, cirrhosis and liver cancer more often than HCV mono-infected patients. Until the present study, there was no suitable in vitro assay to test the inhibitory activity of drugs on HCV/HIV-1 co-infection. Here we developed a novel in vitro "co-infection" model where HCV and HIV-1 concurrently replicate in their respective main host target cells--human hepatocytes and CD4+ T-lymphocytes. Using this co-culture model, we demonstrate that cyclophilin inhibitors (CypI), including a novel cyclosporin A (CsA) analog, CPI-431-32, simultaneously inhibits replication of both HCV and HIV-1 when added pre- and post-infection. In contrast, the HIV-1 protease inhibitor nelfinavir or the HCV NS5A inhibitor daclatasvir only blocks the replication of a single virus in the "co-infection" system. CPI-431-32 efficiently inhibits HCV and HIV-1 variants, which are normally resistant to DAAs. CPI-431-32 is slightly, but consistently more efficacious than the most advanced clinically tested CypI--alisporivir (ALV)--at interrupting an established HCV/HIV-1 co-infection. The superior antiviral efficacy of CPI-431-32 over ALV correlates with its higher potency inhibition of cyclophilin A (CypA) isomerase activity and at preventing HCV NS5A-CypA and HIV-1 capsid-CypA interactions known to be vital for replication of the respective viruses. Moreover, we obtained evidence that CPI-431-32 prevents the cloaking of both the HIV-1 and HCV genomes from cellular sensors. Based on these results, CPI-431-32 has the potential, as a single agent or in combination with DAAs, to inhibit both HCV and HIV-1 infections.
Conflict of interest statement
Figures




Similar articles
-
The combination of alisporivir plus an NS5A inhibitor provides additive to synergistic anti-hepatitis C virus activity without detectable cross-resistance.Antimicrob Agents Chemother. 2014 Jun;58(6):3327-34. doi: 10.1128/AAC.00016-14. Epub 2014 Mar 31. Antimicrob Agents Chemother. 2014. PMID: 24687498 Free PMC article.
-
Characterization of the Anti-HCV Activities of the New Cyclophilin Inhibitor STG-175.PLoS One. 2016 Apr 22;11(4):e0152036. doi: 10.1371/journal.pone.0152036. eCollection 2016. PLoS One. 2016. PMID: 27104614 Free PMC article.
-
The cyclophilin inhibitor SCY-635 disrupts hepatitis C virus NS5A-cyclophilin A complexes.Antimicrob Agents Chemother. 2012 Jul;56(7):3888-97. doi: 10.1128/AAC.00693-12. Epub 2012 May 14. Antimicrob Agents Chemother. 2012. PMID: 22585215 Free PMC article.
-
Cyclophilin inhibitors for the treatment of HCV infection.Curr Opin Investig Drugs. 2010 Aug;11(8):911-8. Curr Opin Investig Drugs. 2010. PMID: 20721833 Review.
-
Profile of alisporivir and its potential in the treatment of hepatitis C.Drug Des Devel Ther. 2013;7:105-15. doi: 10.2147/DDDT.S30946. Epub 2013 Feb 15. Drug Des Devel Ther. 2013. PMID: 23440335 Free PMC article. Review.
Cited by
-
Non-Immunosuppressive Cyclophilin Inhibitors.Angew Chem Int Ed Engl. 2022 Sep 26;61(39):e202201597. doi: 10.1002/anie.202201597. Epub 2022 Aug 19. Angew Chem Int Ed Engl. 2022. PMID: 35290695 Free PMC article. Review.
-
The emerging importance of immunophilins in fibrosis development.Mol Cell Biochem. 2023 Jun;478(6):1281-1291. doi: 10.1007/s11010-022-04591-1. Epub 2022 Oct 27. Mol Cell Biochem. 2023. PMID: 36302992 Free PMC article. Review.
-
Cyclophilin A: promising target in cancer therapy.Cancer Biol Ther. 2024 Dec 31;25(1):2425127. doi: 10.1080/15384047.2024.2425127. Epub 2024 Nov 8. Cancer Biol Ther. 2024. PMID: 39513594 Free PMC article. Review.
-
A Pan-Cyclophilin Inhibitor, CRV431, Decreases Fibrosis and Tumor Development in Chronic Liver Disease Models.J Pharmacol Exp Ther. 2019 Nov;371(2):231-241. doi: 10.1124/jpet.119.261099. Epub 2019 Aug 12. J Pharmacol Exp Ther. 2019. PMID: 31406003 Free PMC article.
-
Cyclosporin A: A Repurposable Drug in the Treatment of COVID-19?Front Med (Lausanne). 2021 Sep 6;8:663708. doi: 10.3389/fmed.2021.663708. eCollection 2021. Front Med (Lausanne). 2021. PMID: 34552938 Free PMC article. Review.
References
-
- Coste J, Reesink HW, Engelfriet CP, Laperche S, Brown S, Busch MP, et al. (2005) Implementation of donor screening for infectious agents transmitted by blood by nucleic acid technology: update to 2003. Vox Sang 88: 289–303. - PubMed
-
- Sulkowski MS, Thomas DL (2003) Hepatitis C in the HIV-Infected Person. Ann Intern Med 138: 197–207. - PubMed
-
- Garten RJ, Lai S, Zhang J, Liu W, Chen J, Vlahov D, et al. (2004) Rapid transmission of hepatitis C virus among young injecting heroin users in Southern China. Int J Epidemiol 33: 182–188. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

