Human iPSC derived disease model of MERTK-associated retinitis pigmentosa
- PMID: 26263531
- PMCID: PMC4531787
- DOI: 10.1038/srep12910
Human iPSC derived disease model of MERTK-associated retinitis pigmentosa
Abstract
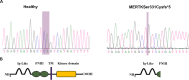
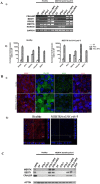
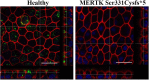
Retinitis pigmentosa (RP) represents a genetically heterogeneous group of retinal dystrophies affecting mainly the rod photoreceptors and in some instances also the retinal pigment epithelium (RPE) cells of the retina. Clinical symptoms and disease progression leading to moderate to severe loss of vision are well established and despite significant progress in the identification of causative genes, the disease pathology remains unclear. Lack of this understanding has so far hindered development of effective therapies. Here we report successful generation of human induced pluripotent stem cells (iPSC) from skin fibroblasts of a patient harboring a novel Ser331Cysfs*5 mutation in the MERTK gene. The patient was diagnosed with an early onset and severe form of autosomal recessive RP (arRP). Upon differentiation of these iPSC towards RPE, patient-specific RPE cells exhibited defective phagocytosis, a characteristic phenotype of MERTK deficiency observed in human patients and animal models. Thus we have created a faithful cellular model of arRP incorporating the human genetic background which will allow us to investigate in detail the disease mechanism, explore screening of a variety of therapeutic compounds/reagents and design either combined cell and gene- based therapies or independent approaches.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

