LTBP-2 Has a Single High-Affinity Binding Site for FGF-2 and Blocks FGF-2-Induced Cell Proliferation
- PMID: 26263555
- PMCID: PMC4532469
- DOI: 10.1371/journal.pone.0135577
LTBP-2 Has a Single High-Affinity Binding Site for FGF-2 and Blocks FGF-2-Induced Cell Proliferation
Abstract
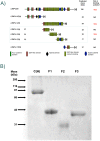
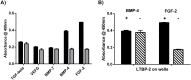
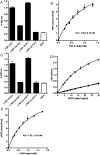
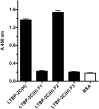
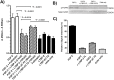
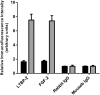
Latent transforming growth factor-beta-1 binding protein-2 (LTBP-2) belongs to the fibrillin-LTBP superfamily of extracellular matrix proteins. LTBPs and fibrillins are involved in the sequestration and storage of latent growth factors, particularly transforming growth factor β (TGF-β), in tissues. Unlike other LTBPs, LTBP-2 does not covalently bind TGF-β, and its molecular functions remain unclear. We are screening LTBP-2 for binding to other growth factors and have found very strong saturable binding to fibroblast growth factor-2 (FGF-2) (Kd = 1.1 nM). Using a series of recombinant LTBP-2 fragments a single binding site for FGF-2 was identified in a central region of LTBP-2 consisting of six tandem epidermal growth factor-like (EGF-like) motifs (EGFs 9-14). This region was also shown to contain a heparin/heparan sulphate-binding site. FGF-2 stimulation of fibroblast proliferation was completely negated by the addition of 5-fold molar excess of LTBP-2 to the assay. Confocal microscopy showed strong co-localisation of LTBP-2 and FGF-2 in fibrotic keloid tissue suggesting that the two proteins may interact in vivo. Overall the study indicates that LTBP-2 is a potent inhibitor of FGF-2 that may influence FGF-2 bioactivity during wound repair particularly in fibrotic tissues.
Conflict of interest statement
Figures



References
-
- Hyytiainen M, Penttinen C, Keski-Oja J (2004) Latent TGF-beta binding proteins: extracellular matrix association and roles in TGF-beta activation. Crit Rev Clin Lab Sci 41: 233–264. - PubMed
-
- Kielty CM, Sherratt MJ, Marson A, Baldock C (2005) Fibrillin microfibrils. Adv Protein Chem 70: 405–436. - PubMed
-
- Rifkin DB (2005) Latent transforming growth factor-beta (TGF-beta) binding proteins: orchestrators of TGF-beta availability. J Biol Chem 280: 7409–7412. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

