Brain-Specific Cytoskeletal Damage Markers in Cerebrospinal Fluid: Is There a Common Pattern between Amyotrophic Lateral Sclerosis and Primary Progressive Multiple Sclerosis?
- PMID: 26263977
- PMCID: PMC4581209
- DOI: 10.3390/ijms160817565
Brain-Specific Cytoskeletal Damage Markers in Cerebrospinal Fluid: Is There a Common Pattern between Amyotrophic Lateral Sclerosis and Primary Progressive Multiple Sclerosis?
Abstract
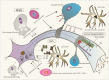
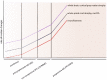
Many neurodegenerative disorders share a common pathophysiological pathway involving axonal degeneration despite different etiological triggers. Analysis of cytoskeletal markers such as neurofilaments, protein tau and tubulin in cerebrospinal fluid (CSF) may be a useful approach to detect the process of axonal damage and its severity during disease course. In this article, we review the published literature regarding brain-specific CSF markers for cytoskeletal damage in primary progressive multiple sclerosis and amyotrophic lateral sclerosis in order to evaluate their utility as a biomarker for disease progression in conjunction with imaging and histological markers which might also be useful in other neurodegenerative diseases associated with affection of the upper motor neurons. A long-term benefit of such an approach could be facilitating early diagnostic and prognostic tools and assessment of treatment efficacy of disease modifying drugs.
Keywords: amyotrophic lateral sclerosis (ALS); biomarker; neurofilaments; primary progressive multiple sclerosis (PPMS); tau; tubulin.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

