Antiandrogen Therapy with Hydroxyflutamide or Androgen Receptor Degradation Enhancer ASC-J9 Enhances BCG Efficacy to Better Suppress Bladder Cancer Progression
- PMID: 26264279
- PMCID: PMC4704455
- DOI: 10.1158/1535-7163.MCT-14-1055-T
Antiandrogen Therapy with Hydroxyflutamide or Androgen Receptor Degradation Enhancer ASC-J9 Enhances BCG Efficacy to Better Suppress Bladder Cancer Progression
Abstract
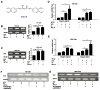
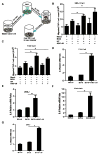
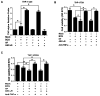
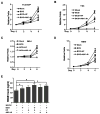
Recent studies suggest that the androgen receptor (AR) might play important roles in influencing bladder cancer progression, yet its clinical application remains unclear. Here, we developed a new combined therapy with Bacillus Calmette-Guérin (BCG) and the AR degradation enhancer ASC-J9 or antiandrogen hydroxyflutamide (HF) to better suppress bladder cancer progression. Mechanism dissection revealed that ASC-J9 treatment enhanced BCG efficacy to suppress bladder cancer cell proliferation via increasing the recruitment of monocytes/macrophages that involved the promotion of BCG attachment/internalization to the bladder cancer cells through increased integrin-α5β1 expression and IL6 release. Such consequences might then enhance BCG-induced bladder cancer cell death via increased TNFα release. Interestingly, we also found that ASC-J9 treatment could directly promote BCG-induced HMGB1 release to enhance the BCG cytotoxic effects for suppression of bladder cancer cell growth. In vivo approaches also concluded that ASC-J9 could enhance the efficacy of BCG to better suppress bladder cancer progression in BBN-induced bladder cancer mouse models. Together, these results suggest that the newly developed therapy combining BCG plus ASC-J9 may become a novel therapy to better suppress bladder cancer progress.
©2015 American Association for Cancer Research.
Conflict of interest statement
Conflicts of interest: ASC-J9® was patented by the University of Rochester, University of North Carolina, and AndroScience, and then licensed to AndroScience. Both the University of Rochester and C.C. own royalties and equity in AndroScience.
Figures
References
-
- Siegel R1, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014 Jan-Feb;64(1):9–29. - PubMed
-
- Morales A, Eidinger D, Bruce AW. Intracavitary bacillus Calmette-Guerin in the treatment of superficial bladder tumors. J Urol. 1976;116:180–3. - PubMed
-
- Huben RP. Intravesical therapeutic versus immunotherapy for superficial bladder cancer. Semin Urol Oncol. 1996;14(1):17–22. - PubMed
-
- Alexandroff AB, Jackson AM, O’Donnell MA, James K. BCG immunotherapy of bladder cancer: 20 years on. Lancet. 1999;353:1689–1694. - PubMed
-
- Miyamoto H, Yang Z, Chen YT, Ishiguro H, Uemura H, Kubota Y, et al. Promotion of Bladder Cancer Development and Progression by Androgen Receptor Signals. JNCI. 2007;99:558–68. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

