Safe and bodywide muscle transduction in young adult Duchenne muscular dystrophy dogs with adeno-associated virus
- PMID: 26264580
- PMCID: PMC4581611
- DOI: 10.1093/hmg/ddv310
Safe and bodywide muscle transduction in young adult Duchenne muscular dystrophy dogs with adeno-associated virus
Abstract
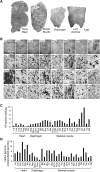
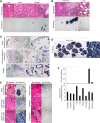
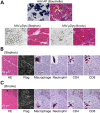
The ultimate goal of muscular dystrophy gene therapy is to treat all muscles in the body. Global gene delivery was demonstrated in dystrophic mice more than a decade ago using adeno-associated virus (AAV). However, translation to affected large mammals has been challenging. The only reported attempt was performed in newborn Duchenne muscular dystrophy (DMD) dogs. Unfortunately, AAV injection resulted in growth delay, muscle atrophy and contracture. Here we report safe and bodywide AAV delivery in juvenile DMD dogs. Three ∼2-m-old affected dogs received intravenous injection of a tyrosine-engineered AAV-9 reporter or micro-dystrophin (μDys) vector at the doses of 1.92-6.24 × 10(14) viral genome particles/kg under transient or sustained immune suppression. DMD dogs tolerated injection well and their growth was not altered. Hematology and blood biochemistry were unremarkable. No adverse reactions were observed. Widespread muscle transduction was seen in skeletal muscle, the diaphragm and heart for at least 4 months (the end of the study). Nominal expression was detected in internal organs. Improvement in muscle histology was observed in μDys-treated dogs. In summary, systemic AAV gene transfer is safe and efficient in young adult dystrophic large mammals. This may translate to bodywide gene therapy in pediatric patients in the future.
© The Author 2015. Published by Oxford University Press. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures


References
-
- Mingozzi F., High K.A. (2011) Therapeutic in vivo gene transfer for genetic disease using AAV: progress and challenges. Nat. Rev. Genet., 12, 341–355. - PubMed
-
- Lewis R. (2014) Gene therapy's second act. Sci. Am., 310, 52–57. - PubMed
-
- Wilson J.M. (2009) Lessons learned from the gene therapy trial for ornithine transcarbamylase deficiency. Mol. Genet. Metab., 96, 151–157. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

