Drug-releasing mesenchymal cells strongly suppress B16 lung metastasis in a syngeneic murine model
- PMID: 26264809
- PMCID: PMC4534150
- DOI: 10.1186/s13046-015-0200-3
Drug-releasing mesenchymal cells strongly suppress B16 lung metastasis in a syngeneic murine model
Abstract
Background: Mesenchymal stromal cells (MSCs) are considered an important therapeutic tool in cancer therapy. They possess intrinsic therapeutic potential and can also be in vitro manipulated and engineered to produce therapeutic molecules that can be delivered to the site of diseases, through their capacity to home pathological tissues. We have recently demonstrated that MSCs, upon in vitro priming with anti-cancer drug, become drug-releasing mesenchymal cells (Dr-MCs) able to strongly inhibit cancer cells growth.
Methods: Murine mesenchymal stromal cells were loaded with Paclitaxel (Dr-MCsPTX) according to a standardized procedure and their ability to inhibit the growth of a murine B16 melanoma was verified by in vitro assays. The anti-metastatic activity of Dr-MCsPTX was then studied in mice injected i.v. with B16 melanoma cells that produced lung metastatic nodules. Lung nodules were counted under a dissecting stereomicroscope and metastasis investigated by histological analysis.
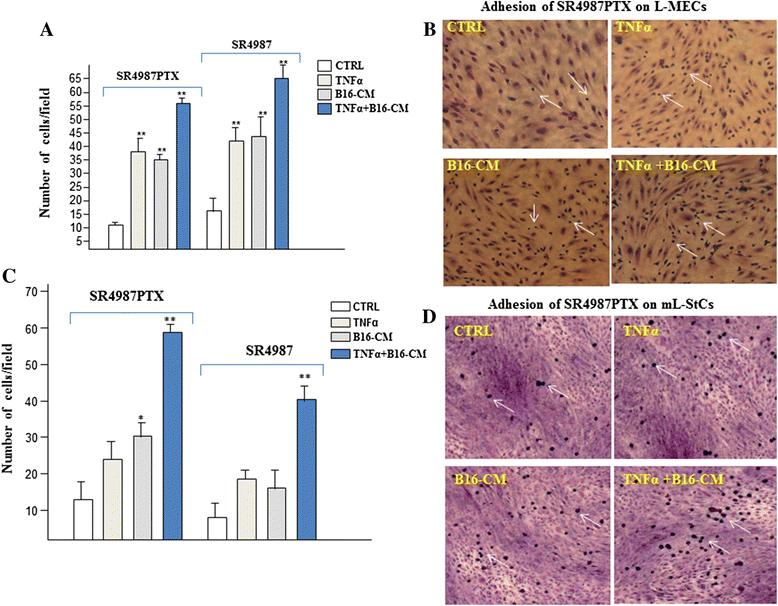
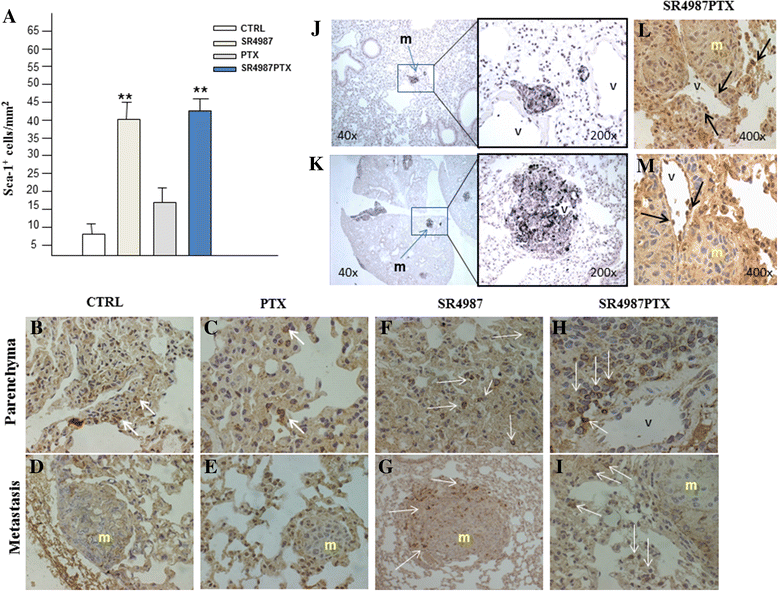
Results: We found that three i.v. injections of Dr-MCsPTX on day 5, 10 and 15 after tumor injection almost completely abolished B16 lung metastasis. Dr-MCsPTX arrested into lung by interacting with endothelium and migrate toward cancer nodule through a complex mechanism involving primarily mouse lung stromal cells (mL-StCs) and SDF-1/CXCR4/CXCR7 axis.
Conclusions: Our results show for the first time that Dr-MCsPTX are very effective to inhibit lung metastasis formation. Actually, a cure for lung metastasis in humans is mostly unlikely and we do not know whether a therapy combining engineered MSCs and Dr-MCs may work synergistically. However, we think that our approach using Dr-MCs loaded with PTX may represent a new valid and additive therapeutic tool to fight lung metastases and, perhaps, primary lung cancers in human.
Figures



Similar articles
-
Phyto-sesquiterpene lactone deoxyelephantopin and cisplatin synergistically suppress lung metastasis of B16 melanoma in mice with reduced nephrotoxicity.Phytomedicine. 2019 Mar 15;56:194-206. doi: 10.1016/j.phymed.2018.11.005. Epub 2018 Nov 6. Phytomedicine. 2019. PMID: 30668340
-
Ex vivo and in vivo delivery of anti-tissue factor short interfering RNA inhibits mouse pulmonary metastasis of B16 melanoma cells.Clin Cancer Res. 2006 Jul 1;12(13):4055-61. doi: 10.1158/1078-0432.CCR-05-2482. Clin Cancer Res. 2006. PMID: 16818705
-
Syngeneic murine metastasis models: B16 melanoma.Methods Mol Biol. 2014;1070:131-40. doi: 10.1007/978-1-4614-8244-4_10. Methods Mol Biol. 2014. PMID: 24092437
-
Expression of CXC chemokine receptor-4 enhances the pulmonary metastatic potential of murine B16 melanoma cells.Cancer Res. 2002 Dec 15;62(24):7328-34. Cancer Res. 2002. PMID: 12499276
-
Influence of mesenchymal stem cells on metastasis development in mice in vivo.Stem Cell Res Ther. 2015 Feb 27;6(1):15. doi: 10.1186/s13287-015-0003-7. Stem Cell Res Ther. 2015. PMID: 25888992 Free PMC article.
Cited by
-
Adipose-derived stem cell-mediated paclitaxel delivery inhibits breast cancer growth.PLoS One. 2018 Sep 7;13(9):e0203426. doi: 10.1371/journal.pone.0203426. eCollection 2018. PLoS One. 2018. PMID: 30192811 Free PMC article.
-
Bone marrow stromal cells from β-thalassemia patients have impaired hematopoietic supportive capacity.J Clin Invest. 2019 Feb 25;129(4):1566-1580. doi: 10.1172/JCI123191. eCollection 2019 Feb 25. J Clin Invest. 2019. PMID: 30830876 Free PMC article.
-
Therapeutic Mesenchymal Stromal Cells for Immunotherapy and for Gene and Drug Delivery.Mol Ther Methods Clin Dev. 2020 Jan 22;16:204-224. doi: 10.1016/j.omtm.2020.01.005. eCollection 2020 Mar 13. Mol Ther Methods Clin Dev. 2020. PMID: 32071924 Free PMC article. Review.
-
The Challenging Treatment of Cisplatin-Resistant Tumors: State of the Art and Future Perspectives.Molecules. 2023 Apr 12;28(8):3407. doi: 10.3390/molecules28083407. Molecules. 2023. PMID: 37110640 Free PMC article. Review.
-
Engineered Mesenchymal Stem Cells as Treatment for Cancers: Opportunities, Clinical Applications and Challenges.Malays J Med Sci. 2024 Oct;31(5):56-82. doi: 10.21315/mjms2024.31.5.5. Epub 2024 Oct 8. Malays J Med Sci. 2024. PMID: 39416732 Free PMC article. Review.
References
-
- Studeny M, Marini FC, Champlin RE, Zompetta C, Fidler IJ, Andreef M. Bone marrow-derived mesenchymal stem cells as vehicles for interferon-beta delivery into tumors. Cancer Res. 2002;62:3603–3608. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

