Global Analysis of Protein Expression and Phosphorylation Levels in Nicotine-Treated Pancreatic Stellate Cells
- PMID: 26265067
- PMCID: PMC4868188
- DOI: 10.1021/acs.jproteome.5b00398
Global Analysis of Protein Expression and Phosphorylation Levels in Nicotine-Treated Pancreatic Stellate Cells
Abstract
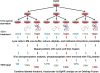
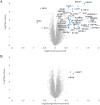
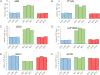
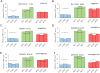
Smoking is a risk factor in pancreatic disease; however, the biochemical mechanisms correlating smoking with pancreatic dysfunction remain poorly understood. Strategies using multiplexed isobaric tag-based mass spectrometry facilitate the study of drug-induced perturbations on biological systems. Here, we present the first large-scale analysis of the proteomic and phosphoproteomic alterations in pancreatic stellate cells following treatment with two nicotinic acetylcholine receptor (nAChR) ligands: nicotine and α-bungarotoxin. We treated cells with nicotine or α-bungarotoxin for 12 h in triplicate and compared alterations in protein expression and phosphorylation levels to mock-treated cells using a tandem mass tag (TMT9plex)-based approach. Over 8100 proteins were quantified across all nine samples, of which 46 were altered in abundance upon treatment with nicotine. Proteins with increased abundance included those associated with neurons, defense mechanisms, indicators of pancreatic disease, and lysosomal proteins. In addition, we measured differences for ∼16 000 phosphorylation sites across all nine samples using a titanium dioxide-based strategy, of which 132 sites were altered with nicotine and 451 with α-bungarotoxin treatment. Many altered phosphorylation sites were involved in nuclear function and transcriptional events. This study supports the development of future targeted investigations to establish a better understanding for the role of nicotine and associated receptors in pancreatic disease.
Keywords: Fusion; SPS; Tandem mass tags; multiplexing; pancreatic cancer; pancreatitis; phosphopeptide enrichment; synchronous precursor selection.
Conflict of interest statement
Figures


References
-
- Yadav D, Hawes RH, Brand RE, Anderson MA, Money ME, Banks PA, Bishop MD, Baillie J, Sherman S, DiSario J, Burton FR, Gardner TB, Amann ST, Gelrud A, Lawrence C, Elinoff B, Greer JB, O'Connell M, Barmada MM, Slivka A, Whitcomb DC. Alcohol consumption, cigarette smoking, and the risk of recurrent acute and chronic pancreatitis. Arch Intern Med. 2009;169(11):1035–45. - PMC - PubMed
-
- Law R, Parsi M, Lopez R, Zuccaro G, Stevens T. Cigarette smoking is independently associated with chronic pancreatitis. Pancreatology. 2010;10(1):54–9. - PubMed
-
- Lin Y, Tamakoshi A, Hayakawa T, Ogawa M, Ohno Y. Cigarette smoking as a risk factor for chronic pancreatitis: a case-control study in Japan. Research Committee on Intractable Pancreatic Diseases. Pancreas. 2000;21(2):109–14. - PubMed
-
- National Toxicology P. Tobacco-related exposures: tobacco smoking. Report on carcinogens : carcinogen profiles / US Dept of Health and Human Services, Public Health Service, National Toxicology Program. 2011;12:408–410. - PubMed
-
- Prokopczyk B, Hoffmann D, Bologna M, Cunningham AJ, Trushin N, Akerkar S, Boyiri T, Amin S, Desai D, Colosimo S, Pittman B, Leder G, Ramadani M, Henne-Bruns D, Beger HG, El-Bayoumy K. Identification of tobacco-derived compounds in human pancreatic juice. Chem Res Toxicol. 2002;15(5):677–85. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

