Impact of Risk Assessment and Tailored versus Nontailored Risk Information on Colorectal Cancer Testing in Primary Care: A Randomized Controlled Trial
- PMID: 26265201
- PMCID: PMC4592452
- DOI: 10.1158/1055-9965.EPI-15-0122
Impact of Risk Assessment and Tailored versus Nontailored Risk Information on Colorectal Cancer Testing in Primary Care: A Randomized Controlled Trial
Abstract
Background: Colorectal cancer screening is effective but underused. Guidelines for which tests are recommended and at what intervals depend on specific risks. We developed a tablet-based Cancer Risk Intake System (CRIS) that asks questions about risk prior to appointments and generates tailored printouts for patients and physicians summarizing and matching risk factors with guideline-based recommendations.
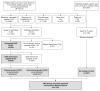
Methods: Randomized controlled trial among patients who: (i) used CRIS and they and their physicians received tailored printouts; (ii) used CRIS to answer questions but received standard information about cancer screening while their physicians received a standard electronic chart prompt indicating they were age-eligible but not currently adherent for colorectal cancer screening; or (iii) comprised a no-contact group that neither used CRIS nor received any information while their physicians received the standard prompt. Participation in testing was assessed via electronic medical record at 12 months.
Results: Participation in any colorectal cancer testing was three times higher for those who used the CRIS and received any printed materials, compared with no-contact controls (47% vs. 16%; P < 0.0001). Among CRIS users ages 50 and older, participation in any testing was higher in the tailored group (53% vs. 44%, P = 0.023).
Conclusion: Use of CRIS and receipt of any information facilitated participation in testing. There was more testing participation in the CRIS-tailored than nontailored group.
Impact: Asking patients questions about their specific risk factors and giving them and their providers information just prior to an appointment may increase participation in colorectal cancer testing. Tailoring the information has some added benefit.
©2015 American Association for Cancer Research.
Conflict of interest statement
Dr. Gupta has received consulting fees from Exact Sciences. No conflicts of interest have been declared by any other authors.
References
-
- Levin B, Lieberman DA, McFarland B, Andrews KS, Brooks D, Bond J, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology. 2008;134:1570–95. - PubMed
-
- U S. Preventive Services Task Force. Screening for Colorectal Cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2008;149:627–38. - PubMed
-
- Smith RA, Manassaram-Baptiste D, Brooks D, Cokkinides V, Doroshenk M, Saslow D, et al. Cancer screening in the United States, 2014: a review of current American Cancer Society guidelines and current issues in cancer screening. CA: A Cancer Journal for Clinicians. 2014;64:30–51. - PubMed
-
- Holden DJ, Jonas DE, Porterfield DS, Reuland D, Harris R. Systematic review: enhancing the use and quality of colorectal cancer screening. Ann Intern Med. 2010;152:668–76. - PubMed
-
- Centers for Disease Control and Prevention. Use of colorectal cancer tests - United States, 2002, 2004, and 2006. MMWR Morb Mortal Wkly Rep. 2008;57:253–8. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical