Myosin VI and cardiomyopathy: Left ventricular hypertrophy, fibrosis, and both cardiac and pulmonary vascular endothelial cell defects in the Snell's waltzer mouse
- PMID: 26265212
- PMCID: PMC4600664
- DOI: 10.1002/cm.21236
Myosin VI and cardiomyopathy: Left ventricular hypertrophy, fibrosis, and both cardiac and pulmonary vascular endothelial cell defects in the Snell's waltzer mouse
Abstract
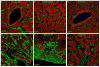
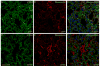
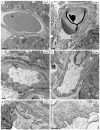
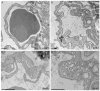
In mice and humans, loss of myosin VI (Myo6) function results in deafness, and certain Myo6 mutations also result in cardiomyopathies in humans. The current studies have utilized the Snell's waltzer (sv) mouse (a functional null mutation for Myo6) to determine if this mouse also exhibits cardiac defects and thus used to determine the cellular and molecular basis for Myo6-associated heart disease. Myo6 is expressed in mouse heart where it is predominantly expressed in vascular endothelial cells (VECs) based on co-localization with the VEC cell marker CD31. Sv/sv heart mass is significantly greater than that of sv/+ littermates, a result of left ventricle hypertrophy. The left ventricle of the sv/sv exhibits extensive fibrosis, both interstitial and perivascular, based on histologic staining, and immunolocalization of several markers for fibrosis including fibronectin, collagen IV, and the fibroblast marker vimentin. Myo6 is also expressed in lung VECs but not in VECs of intestine, kidney, or liver. Sv/sv lungs exhibit increased periaveolar fibrosis and enlarged air sacs. Electron microscopy of sv/sv cardiac and lung VECs revealed abnormal ultrastructure, including luminal protrusions and increased numbers of cytoplasmic vesicles. Previous studies have shown that loss of function of either Myo6 or its adaptor binding partner synectin/GIPC results in impaired arterial development due to defects in VEGF signaling. However, examination of synectin/GIPC-/- heart revealed no fibrosis or significantly altered VEC ultrastructure, suggesting that the cardiac and lung defects observed in the sv/sv mouse are not due to Myo6 function in arterial development.
Keywords: GIPC; Myo6; caveoli; fibrosis; vascular endothelial cell.
© 2015 Wiley Periodicals, Inc.
Figures










References
-
- Ameen N, Apodaca G. Defective CFTR apical endocytosis and enterocyte brush border in myosin VI-deficient mice. Traffic. 2007;8(8):998–1006. - PubMed
-
- Avraham KB, Hasson T, Steel KP, Kingsley DM, Russell LB, Mooseker MS, Copeland NG, Jenkins NA. The mouse Snell’s waltzer deafness gene encodes an unconventional myosin required for structural integrity of inner ear hair cells. Nat Genet. 1995;11(4):369–75. - PubMed
-
- Carmeliet P, Collen D. Transgenic mouse models in angiogenesis and cardiovascular disease. J Pathol. 2000;190(3):387–405. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

