Bacterial rotary export ATPases are allosterically regulated by the nucleotide second messenger cyclic-di-GMP
- PMID: 26265469
- PMCID: PMC4591828
- DOI: 10.1074/jbc.M115.661439
Bacterial rotary export ATPases are allosterically regulated by the nucleotide second messenger cyclic-di-GMP
Abstract
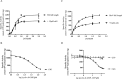
The widespread second messenger molecule cyclic di-GMP (cdG) regulates the transition from motile and virulent lifestyles to sessile, biofilm-forming ones in a wide range of bacteria. Many pathogenic and commensal bacterial-host interactions are known to be controlled by cdG signaling. Although the biochemistry of cyclic dinucleotide metabolism is well understood, much remains to be discovered about the downstream signaling pathways that induce bacterial responses upon cdG binding. As part of our ongoing research into the role of cdG signaling in plant-associated Pseudomonas species, we carried out an affinity capture screen for cdG binding proteins in the model organism Pseudomonas fluorescens SBW25. The flagella export AAA+ ATPase FliI was identified as a result of this screen and subsequently shown to bind specifically to the cdG molecule, with a KD in the low micromolar range. The interaction between FliI and cdG appears to be very widespread. In addition to FliI homologs from diverse bacterial species, high affinity binding was also observed for the type III secretion system homolog HrcN and the type VI ATPase ClpB2. The addition of cdG was shown to inhibit FliI and HrcN ATPase activity in vitro. Finally, a combination of site-specific mutagenesis, mass spectrometry, and in silico analysis was used to predict that cdG binds to FliI in a pocket of highly conserved residues at the interface between two FliI subunits. Our results suggest a novel, fundamental role for cdG in controlling the function of multiple important bacterial export pathways, through direct allosteric control of export ATPase proteins.
Keywords: ATPase; Pseudomonas; bacterial signal transduction; cyclic di-GMP (c-di-GMP); flagellum; second messenger; type III secretion system (T3SS).
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures







References
-
- Naseby D. C., Way J. A., Bainton N. J., Lynch J. M. (2001) Biocontrol of Pythium in the pea rhizosphere by antifungal metabolite producing and non-producing Pseudomonas strains. J. Appl. Microbiol. 90, 421–429 - PubMed
-
- Haas D., Défago G. (2005) Biological control of soil-borne pathogens by fluorescent pseudomonads. Nat. Rev. Microbiol. 3, 307–319 - PubMed
-
- Compant S., Clement C., Sessitsch A. (2010) Plant growth-promoting bacteria in the rhizo- and endosphere of plants: their role, colonization, mechnaisms onvolved and prospects for utilization. Soil Biol. Biochem. 42, 669–678
-
- Xin X. F., He S. Y. (2013) Pseudomonas syringae pv. tomato DC3000: a model pathogen for probing disease susceptibility and hormone signaling in plants. Annu. Rev. Phytopathol. 51, 473–498 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Research Materials

