Efficient Endocytic Uptake and Maturation in Drosophila Oocytes Requires Dynamitin/p50
- PMID: 26265702
- PMCID: PMC4596674
- DOI: 10.1534/genetics.115.180018
Efficient Endocytic Uptake and Maturation in Drosophila Oocytes Requires Dynamitin/p50
Abstract
Dynactin is a multi-subunit complex that functions as a regulator of the Dynein motor. A central component of this complex is Dynamitin/p50 (Dmn). Dmn is required for endosome motility in mammalian cell lines. However, the extent to which Dmn participates in the sorting of cargo via the endosomal system is unknown. In this study, we examined the endocytic role of Dmn using the Drosophila melanogaster oocyte as a model. Yolk proteins are internalized into the oocyte via clathrin-mediated endocytosis, trafficked through the endocytic pathway, and stored in condensed yolk granules. Oocytes that were depleted of Dmn contained fewer yolk granules than controls. In addition, these oocytes accumulated numerous endocytic intermediate structures. Particularly prominent were enlarged endosomes that were relatively devoid of Yolk proteins. Ultrastructural and genetic analyses indicate that the endocytic intermediates are produced downstream of Rab5. Similar phenotypes were observed upon depleting Dynein heavy chain (Dhc) or Lis1. Dhc is the motor subunit of the Dynein complex and Lis1 is a regulator of Dynein activity. We therefore propose that Dmn performs its function in endocytosis via the Dynein motor. Consistent with a role for Dynein in endocytosis, the motor colocalized with the endocytic machinery at the oocyte cortex in an endocytosis-dependent manner. Our results suggest a model whereby endocytic activity recruits Dynein to the oocyte cortex. The motor along with its regulators, Dynactin and Lis1, functions to ensure efficient endocytic uptake and maturation.
Keywords: cell polarity; dynactin; endocytosis; kinesin; microtubule motors.
Copyright © 2015 by the Genetics Society of America.
Figures

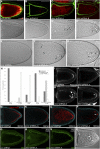




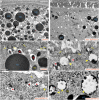


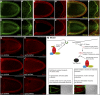
References
-
- Behnia R., Munro S., 2005. Organelle identity and the signposts for membrane traffic. Nature 438: 597–604. - PubMed
-
- Chavrier P., Parton R. G., Hauri H. P., Simons K., Zerial M., 1990. Localization of low molecular weight GTP binding proteins to exocytic and endocytic compartments. Cell 62: 317–329. - PubMed
-
- Chen M. S., Obar R. A., Schroeder C. C., Austin T. W., Poodry C. A., et al. , 1991. Multiple forms of dynamin are encoded by shibire, a Drosophila gene involved in endocytosis. Nature 351: 583–586. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

