Knockout of STAT3 in skeletal muscle does not prevent high-fat diet-induced insulin resistance
- PMID: 26266089
- PMCID: PMC4529495
- DOI: 10.1016/j.molmet.2015.05.001
Knockout of STAT3 in skeletal muscle does not prevent high-fat diet-induced insulin resistance
Abstract
Objective: Increased signal transducer and activator of transcription 3 (STAT3) signaling has been implicated in the development of skeletal muscle insulin resistance, though its contribution, in vivo, remains to be fully defined. Therefore, the aim of this study was to determine whether knockout of skeletal muscle STAT3 would prevent high-fat diet (HFD)-induced insulin resistance.
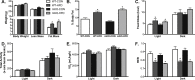
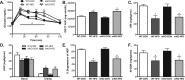
Methods: We used Cre-LoxP methodology to generate mice with muscle-specific knockout (KO) of STAT3 (mKO). Beginning at 10 weeks of age, mKO mice and their wildtype/floxed (WT) littermates either continued consuming a low fat, control diet (CON; 10% of calories from fat) or were switched to a HFD (60% of calories from fat) for 20 days. We measured body composition, energy expenditure, oral glucose tolerance and in vivo insulin action using hyperinsulinemic-euglycemic clamps. We also measured insulin sensitivity in isolated soleus and extensor digitorum longus muscles using the 2-deoxy-glucose (2DOG) uptake technique.
Results: STAT3 protein expression was reduced ∼75-100% in muscle from mKO vs. WT mice. Fat mass and body fat percentage did not differ between WT and mKO mice on CON and were increased equally by HFD. There were also no genotype differences in energy expenditure or whole-body fat oxidation. As determined, in vivo (hyperinsulinemic-euglycemic clamps) and ex vivo (2DOG uptake), skeletal muscle insulin sensitivity did not differ between CON-fed mice, and was impaired similarly by HFD.
Conclusions: These results demonstrate that STAT3 activation does not underlie the development of HFD-induced skeletal muscle insulin resistance.
Keywords: 2DOG, 2-deoxyglucose; AT, adipose tissue; Adgre1, adhesion G protein-coupled receptor E1; CON, normal chow, control diet; Clamp; Cre-LoxP; EDL, extensor digitorum longus; GA, gastrocnemius; GIR, glucose infusion rate; Glucose homeostasis; HFD, high-fat diet; HGP, hepatic glucose production; HYP-EUG, hyperinsulinemic-euglycemic; IL, interleukin; IS-GDR, insulin-stimulated glucose disposal rate; In vivo; KO, knockout; MCK, muscle creatine kinase; Obesity; STAT3; STAT3, signal transducer and activator of transcription 3; T2D, type 2 diabetes; WT, wild-type; mKO, muscle-specific knockout of STAT3.
Figures


References
-
- Osborn O., Olefsky J.M. The cellular and signaling networks linking the immune system and metabolism in disease. Nature Medicine. 2012;18:363–374. - PubMed
-
- Galic S., Sachithanandan N., Kay T.W., Steinberg G.R. Suppressor of cytokine signalling (SOCS) proteins as guardians of inflammatory responses critical for regulating insulin sensitivity. Biochemical Journal. 2014;461:177–188. - PubMed
-
- Kristiansen O.P., Mandrup-Poulsen T. Interleukin-6 and diabetes: the good, the bad, or the indifferent? Diabetes. 2005;54(Suppl 2):S114–S124. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

