Uncontrolled sepsis: a systematic review of translational immunology studies in intensive care medicine
- PMID: 26266907
- PMCID: PMC4513024
- DOI: 10.1186/2197-425X-2-6
Uncontrolled sepsis: a systematic review of translational immunology studies in intensive care medicine
Abstract
Background: The design of clinical immunology studies in sepsis presents several fundamental challenges to improving the translational understanding of pathologic mechanisms. We undertook a systematic review of bed-to-benchside studies to test the hypothesis that variable clinical design methodologies used to investigate immunologic function in sepsis contribute to apparently conflicting laboratory data, and identify potential alternatives that overcome various obstacles to improve experimental design.
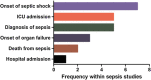
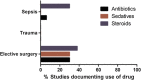
Methods: We performed a systematic review of the design methodology employed to study neutrophil function (respiratory burst), monocyte endotoxin tolerance and lymphocyte apoptosis in the intensive care setting, over the past 15 years. We specifically focussed on how control samples were defined, taking into account age, gender, ethnicity, concomitant therapies, timing of sample collection and the criteria used to diagnose sepsis.
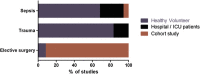
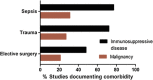
Results: We identified 57 eligible studies, the majority of which (74%) used case-control methodology. Healthy volunteers represented the control population selected in 83% of studies. Comprehensive demographic data on age, gender and ethnicity were provided in ≤48% of case control studies. Documentation of diseases associated with immunosuppression, malignancy and immunomodulatory therapies was rare. Less than half (44%) of studies undertook independent adjudication for the diagnosis of sepsis while 68% provided microbiological data. The timing of sample collection was defined by highly variable clinical criteria. By contrast, surgical studies avoided many such confounders, although only one study in surgical patients monitored the study group for development of sepsis.
Conclusions: We found several important and common limitations in the clinical design of translational immunologic studies in human sepsis. Major elective surgery overcame many of these methodological limitations. The failure of adequate clinical design in mechanistic studies may contribute to the lack of translational therapeutic progress in intensive care medicine.
Figures

References
-
- Ranieri VM, Thompson BT, Barie PS, Dhainaut J-F, Douglas IS, Finfer S, Gårdlund B, Marshall JC, Rhodes A, Artigas A, Payen D, Tenhunen J, Al-Khalidi HR, Thompson V, Janes J, Macias WL, Vangerow B, Williams MD. Drotrecogin Alfa (activated) in adults with septic shock. N Engl J Med. 2012;366:2055–2064. doi: 10.1056/NEJMoa1202290. - DOI - PubMed
-
- Finfer S, Chittock DR, Su SY-S, Blair D, Foster D, Dhingra V, Bellomo R, Cook D, Dodek P, Henderson WR, Hébert PC, Heritier S, Heyland DK, McArthur C, McDonald E, Mitchell I, Myburgh JA, Norton R, Potter J, Robinson BG, Ronco JJ. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360:1283–1297. doi: 10.1056/NEJMoa0810625. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
