In vitro glycoengineering of IgG1 and its effect on Fc receptor binding and ADCC activity
- PMID: 26266936
- PMCID: PMC4534130
- DOI: 10.1371/journal.pone.0134949
In vitro glycoengineering of IgG1 and its effect on Fc receptor binding and ADCC activity
Abstract
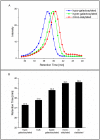
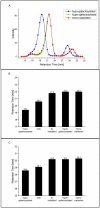
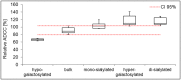
The importance and effect of Fc glycosylation of monoclonal antibodies with regard to biological activity is widely discussed and has been investigated in numerous studies. Fc glycosylation of monoclonal antibodies from current production systems is subject to batch-to-batch variability. If there are glycosylation changes between different batches, these changes are observed not only for one but multiple glycan species. Therefore, studying the effect of distinct Fc glycan species such as galactosylated and sialylated structures is challenging due to the lack of well-defined differences in glycan patterns of samples used. In this study, the influence of IgG1 Fc galactosylation and sialylation on its effector functions has been investigated using five different samples which were produced from one single drug substance batch by in vitro glycoengineering. This sample set comprises preparations with minimal and maximal galactosylation and different levels of sialylation of fully galactosylated Fc glycans. Among others, Roche developed the glycosyltransferase enzyme sialyltransferase which was used for the in vitro glycoengineering activities at medium scale. A variety of analytical assays, including Surface Plasmon Resonance and recently developed FcγR affinity chromatography, as well as an optimized cell-based ADCC assay were applied to investigate the effect of Fc galactosylation and sialylation on the in vitro FcγRI, IIa, and IIIa receptor binding and ADCC activity of IgG1. The results of our studies do not show an impact, neither positive nor negative, of sialic acid- containing Fc glycans of IgG1 on ADCC activity, FcγRI, and RIIIa receptors, but a slightly improved binding to FcγRIIa. Furthermore, we demonstrate a galactosylation-induced positive impact on the binding activity of the IgG1 to FcγRIIa and FcγRIIIa receptors and ADCC activity.
Conflict of interest statement
Figures


References
-
- Pacis E, Yu M, Autsen J, Bayer R, Li F. Effects of cell culture conditions on antibody N-linked glycosylation-what affects high mannose 5 glycoform. Biotechnol Bioeng 2011. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

