L-citrulline production by metabolically engineered Corynebacterium glutamicum from glucose and alternative carbon sources
- PMID: 26267114
- PMCID: PMC4883986
- DOI: 10.1186/s13568-014-0085-0
L-citrulline production by metabolically engineered Corynebacterium glutamicum from glucose and alternative carbon sources
Abstract
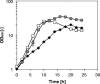
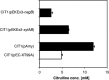
L-citrulline plays an important role in human health and nutrition and is an intermediate of the L-arginine biosynthetic pathway. L-citrulline is a by-product of L-arginine production by Corynebacterium glutamicum. In this study, C. glutamicum was engineered for overproduction of L-citrulline as major product without L-arginine being produced as by-product. To this end, L-arginine biosynthesis was derepressed by deletion of the arginine repressor gene argR and conversion of L-citrulline towards L-arginine was avoided by deletion of the argininosuccinate synthetase gene argG. Moreover, to facilitate L-citrulline production the gene encoding a feedback resistant N-acetyl L-glutamate kinase argB (fbr) as well as the gene encoding L-ornithine carbamoylphosphate transferase argF were overexpressed. The resulting strain accumulated 44.1 ± 0.5 mM L-citrulline from glucose minimal medium with a yield of 0.38 ± 0.01 g[Symbol: see text]g(-1) and a volumetric productivity of 0.32 ± 0.01 g[Symbol: see text]l(-1)[Symbol: see text]h(-1). In addition, production of L-citrulline from the alternative carbon sources starch, xylose, and glucosamine could be demonstrated.
Figures



References
-
- An SJ, Yim SS, Jeong KJ. Development of a secretion system for the production of heterologous proteins in Corynebacterium glutamicum using the Porin B signal peptide. Protein Expr Purif. 2013;89(2):251–257. - PubMed
-
- Arndt A, Eikmanns BJ. Regulation of carbon metabolism in Corynebacterium glutamicum. In: Burkovski A, editor. Corynebacteria: genomics and molecular biology. Wymondham, UK: Caister Academic Press; 2008. pp. 155–182.
-
- Baumgart M, Unthan S, Ruckert C, Sivalingam J, Grunberger A, Kalinowski J, Bott M, Noack S, Frunzke J. Construction of a prophage-free variant of Corynebacterium glutamicum ATCC 13032 for use as a platform strain for basic research and industrial biotechnology. Appl Environ Microbiol. 2013;79(19):6006–6015. - PMC - PubMed
-
- Blombach B, Seibold GM. Carbohydrate metabolism in Corynebacterium glutamicum and applications for the metabolic engineering of L-lysine production strains. Appl Microbiol Biotechnol. 2010;86(5):1313–1322. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

