Effects of a One Year Reusable Contraceptive Vaginal Ring on Vaginal Microflora and the Risk of Vaginal Infection: An Open-Label Prospective Evaluation
- PMID: 26267119
- PMCID: PMC4534458
- DOI: 10.1371/journal.pone.0134460
Effects of a One Year Reusable Contraceptive Vaginal Ring on Vaginal Microflora and the Risk of Vaginal Infection: An Open-Label Prospective Evaluation
Abstract
Background: A contraceptive vaginal ring (CVR) containing Nestorone® (NES) and ethinyl estradiol (EE) that is reusable for 1- year (13 cycles) is under development. This study assessed effects of this investigational CVR on the incidence of vaginal infections and change in vaginal microflora.
Methods: There were 120 women enrolled into a NES/EE CVR Phase III trial and a microbiology sub-study for up to 1- year of cyclic product use. Gynecological examinations were conducted at baseline, the first week of cycle 6 and last week of cycle 13 (or during early discontinuation visits). Vaginal swabs were obtained for wet mount microscopy, Gram stain and culture. The CVR was removed from the vagina at the last study visit and cultured. Semi-quantitative cultures for Lactobacillus, Gardnerella vaginalis, Enterococcus faecalis, Staphylococcus aureus, Escherichia coli, anaerobic gram negative rods (GNRs), Candida albicans and other yeasts were performed on vaginal and CVR samples. Vaginal infections were documented throughout the study.
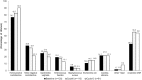
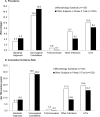
Results: Over 1- year of use, 3.3% of subjects were clinically diagnosed with bacterial vaginosis, 15.0% with vulvovaginal candidiasis, and 0.8% with trichomoniasis. The detection rate of these three infections did not change significantly from baseline to either Cycle 6 or 13. Nugent scores remained stable. H2O2-positive Lactobacillus dominated vaginal flora with a non-significant prevalence increase from 76.7% at baseline to 82.7% at cycle 6 and 90.2% at cycle 13, and a median concentration of 107 colony forming units (cfu) per gram. Although anaerobic GNRs prevalence increased significantly, the median concentration decreased slightly (104 to 103cfu per gram). There were no significant changes in frequency or concentrations of other pathogens. High levels of agreement between vaginal and ring surface microbiota were observed.
Conclusion: Sustained use of the NES/EE CVR did not increase the risk of vaginal infection and was not disruptive to the vaginal ecosystem.
Trial registration: ClinicalTrials.gov NCT00263341, NCT00455156.
Conflict of interest statement
Figures

References
-
- Alkema L, Kantorova V, Menozzi C, Biddlecom A. National, regional, and global rates and trends in contraceptive prevalence and unmet need for family planning between 1990 and 2015: a systematic and comprehensive analysis. Lancet. 2013; 381(9878):1642–1652. 10.1016/S0140-6736(12)62204-1 - DOI - PubMed
-
- Sivin I, Mishell DR Jr, Alvarez F, Brache V, Elomaa K, Lähteenmäki P, et al. Contraceptive vaginal rings releasing Nestorone and ethinylestradiol: a 1-year dose-finding trial. Contraception. 2005; 71(2):122–129. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

