Flow Cytometric Analysis of Myeloid Cells in Human Blood, Bronchoalveolar Lavage, and Lung Tissues
- PMID: 26267148
- PMCID: PMC4742930
- DOI: 10.1165/rcmb.2015-0146OC
Flow Cytometric Analysis of Myeloid Cells in Human Blood, Bronchoalveolar Lavage, and Lung Tissues
Abstract
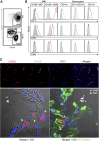
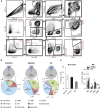
Clear identification of specific cell populations by flow cytometry is important to understand functional roles. A well-defined flow cytometry panel for myeloid cells in human bronchoalveolar lavage (BAL) and lung tissue is currently lacking. The objective of this study was to develop a flow cytometry-based panel for human BAL and lung tissue. We obtained and performed flow cytometry/sorting on human BAL cells and lung tissue. Confocal images were obtained from lung tissue using antibodies for cluster of differentiation (CD)206, CD169, and E cadherin. We defined a multicolor flow panel for human BAL and lung tissue that identifies major leukocyte populations. These include macrophage (CD206(+)) subsets and other CD206(-) leukocytes. The CD206(-) cells include: (1) three monocyte (CD14(+)) subsets, (2) CD11c(+) dendritic cells (CD14(-), CD11c(+), HLA-DR(+)), (3) plasmacytoid dendritic cells (CD14(-), CD11c(-), HLA-DR(+), CD123(+)), and (4) other granulocytes (neutrophils, mast cells, eosinophils, and basophils). Using this panel on human lung tissue, we defined two populations of pulmonary macrophages: CD169(+) and CD169(-) macrophages. In lung tissue, CD169(-) macrophages were a prominent cell type. Using confocal microscopy, CD169(+) macrophages were located in the alveolar space/airway, defining them as alveolar macrophages. In contrast, CD169(-) macrophages were associated with airway/alveolar epithelium, consistent with interstitial-associated macrophages. We defined a flow cytometry panel in human BAL and lung tissue that allows identification of multiple immune cell types and delineates alveolar from interstitial-associated macrophages. This study has important implications for defining myeloid cells in human lung samples.
Keywords: alveolar macrophages; interstitial lung disease; interstitial macrophages; interstitial-associated macrophages.
Figures



Comment in
-
Pulmonary Macrophages: Overlooked and Underappreciated.Am J Respir Cell Mol Biol. 2016 Jan;54(1):1-2. doi: 10.1165/rcmb.2015-0270ED. Am J Respir Cell Mol Biol. 2016. PMID: 26720905 No abstract available.
References
-
- De Rosa SC, Herzenberg LA, Herzenberg LA, Roederer M. 11-color, 13-parameter flow cytometry: identification of human naive T cells by phenotype, function, and T-cell receptor diversity. Nat Med. 2001;7:245–248. - PubMed
-
- Ganesan A, Chattopadhyay PK, Brodie TM, Qin J, Gu W, Mascola JR, Michael NL, Follmann DA, Roederer M Infectious Disease Clinical Research Program HIV Working Group. Immunologic and virologic events in early HIV infection predict subsequent rate of progression. J Infect Dis. 2010;201:272–284. - PMC - PubMed
-
- Freel SA, Lamoreaux L, Chattopadhyay PK, Saunders K, Zarkowsky D, Overman RG, Ochsenbauer C, Edmonds TG, Kappes JC, Cunningham CK, et al. Phenotypic and functional profile of HIV-inhibitory CD8 T cells elicited by natural infection and heterologous prime/boost vaccination. J Virol. 2010;84:4998–5006. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

