Maize Domestication and Anti-Herbivore Defences: Leaf-Specific Dynamics during Early Ontogeny of Maize and Its Wild Ancestors
- PMID: 26267478
- PMCID: PMC4534137
- DOI: 10.1371/journal.pone.0135722
Maize Domestication and Anti-Herbivore Defences: Leaf-Specific Dynamics during Early Ontogeny of Maize and Its Wild Ancestors
Abstract
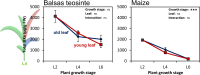
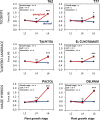
As a consequence of artificial selection for specific traits, crop plants underwent considerable genotypic and phenotypic changes during the process of domestication. These changes may have led to reduced resistance in the cultivated plant due to shifts in resource allocation from defensive traits to increased growth rates and yield. Modern maize (Zea mays ssp. mays) was domesticated from its ancestor Balsas teosinte (Z. mays ssp. parviglumis) approximately 9000 years ago. Although maize displays a high genetic overlap with its direct ancestor and other annual teosintes, several studies show that maize and its ancestors differ in their resistance phenotypes with teosintes being less susceptible to herbivore damage. However, the underlying mechanisms are poorly understood. Here we addressed the question to what extent maize domestication has affected two crucial chemical and one physical defence traits and whether differences in their expression may explain the differences in herbivore resistance levels. The ontogenetic trajectories of 1,4-benzoxazin-3-ones, maysin and leaf toughness were monitored for different leaf types across several maize cultivars and teosinte accessions during early vegetative growth stages. We found significant quantitative and qualitative differences in 1,4-benzoxazin-3-one accumulation in an initial pairwise comparison, but we did not find consistent differences between wild and cultivated genotypes during a more thorough examination employing several cultivars/accessions. Yet, 1,4-benzoxazin-3-one levels tended to decline more rapidly with plant age in the modern maize cultivars. Foliar maysin levels and leaf toughness increased with plant age in a leaf-specific manner, but were also unaffected by domestication. Based on our findings we suggest that defence traits other than the ones that were investigated are responsible for the observed differences in herbivore resistance between teosinte and maize. Furthermore, our results indicate that single pairwise comparisons may lead to false conclusions regarding the effects of domestication on defensive and possibly other traits.
Conflict of interest statement
Figures


Similar articles
-
Maize biochemistry in response to root herbivory was mediated by domestication, spread, and breeding.Planta. 2021 Sep 9;254(4):70. doi: 10.1007/s00425-021-03720-2. Planta. 2021. PMID: 34499214
-
Domestication and lowland adaptation of coastal preceramic maize from Paredones, Peru.Elife. 2023 Apr 18;12:e83149. doi: 10.7554/eLife.83149. Elife. 2023. PMID: 37070964 Free PMC article.
-
Root volatile profiles and herbivore preference are mediated by maize domestication, geographic spread, and modern breeding.Planta. 2022 Dec 23;257(1):24. doi: 10.1007/s00425-022-04057-0. Planta. 2022. PMID: 36562877
-
Genomic screening for artificial selection during domestication and improvement in maize.Ann Bot. 2007 Nov;100(5):967-73. doi: 10.1093/aob/mcm173. Epub 2007 Aug 18. Ann Bot. 2007. PMID: 17704539 Free PMC article. Review.
-
Genomics of Long- and Short-Term Adaptation in Maize and Teosintes.Methods Mol Biol. 2020;2090:289-311. doi: 10.1007/978-1-0716-0199-0_12. Methods Mol Biol. 2020. PMID: 31975172 Review.
Cited by
-
Maize biochemistry in response to root herbivory was mediated by domestication, spread, and breeding.Planta. 2021 Sep 9;254(4):70. doi: 10.1007/s00425-021-03720-2. Planta. 2021. PMID: 34499214
-
A Bird in the Hand Versus Two in the Bush? The Specialist Leafhopper Dalbulus maidis (Hemiptera: Cicadellidae) Does Not Discriminate Against Sub-optimal Host Plants (Zea spp.).Neotrop Entomol. 2018 Apr;47(2):171-180. doi: 10.1007/s13744-017-0516-0. Epub 2017 Apr 10. Neotrop Entomol. 2018. PMID: 28397144
-
Genotypic Variation and Potential Mechanisms of Resistance against Multiple Insect Herbivores in Cranberries.J Chem Ecol. 2024 Nov;50(11):751-766. doi: 10.1007/s10886-024-01522-w. Epub 2024 Jul 19. J Chem Ecol. 2024. PMID: 39028464
-
Integrated IBD Analysis, GWAS Analysis and Transcriptome Analysis to Identify the Candidate Genes for White Spot Disease in Maize.Int J Mol Sci. 2023 Jun 11;24(12):10005. doi: 10.3390/ijms241210005. Int J Mol Sci. 2023. PMID: 37373152 Free PMC article.
-
Herbivory Protection via Volatile Organic Compounds Is Influenced by Maize Genotype, Not Bacillus altitudinis-Enriched Bacterial Communities.Front Microbiol. 2022 May 2;13:826635. doi: 10.3389/fmicb.2022.826635. eCollection 2022. Front Microbiol. 2022. PMID: 35586862 Free PMC article.
References
-
- Benrey B, Callejas A, Rios L, Oyama K, Denno RF. The effects of domestication of Brassica and Phaseolus on the interaction between phytophagous insects and parasitoids. Biological Control. 1998;11(2):130–40. 10.1006/bcon.1997.0590 . - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

