Alzheimer's Disease-Related Protein Expression in the Retina of Octodon degus
- PMID: 26267479
- PMCID: PMC4534194
- DOI: 10.1371/journal.pone.0135499
Alzheimer's Disease-Related Protein Expression in the Retina of Octodon degus
Abstract
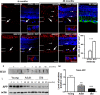
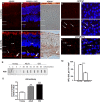
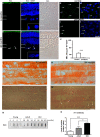
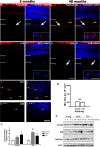
New studies show that the retina also undergoes pathological changes during the development of Alzheimer's disease (AD). While transgenic mouse models used in these previous studies have offered insight into this phenomenon, they do not model human sporadic AD, which is the most common form. Recently, the Octodon degus has been established as a sporadic model of AD. Degus display age-related cognitive impairment associated with Aβ aggregates and phosphorylated tau in the brain. Our aim for this study was to examine the expression of AD-related proteins in young, adult and old degus retina using enzyme-linked or fluorescence immunohistochemistry and to quantify the expression using slot blot and western blot assays. Aβ4G8 and Aβ6E10 detected Aβ peptides in some of the young animals but the expression was higher in the adults. Aβ peptides were observed in the inner and outer segment of the photoreceptors, the nerve fiber layer (NFL) and ganglion cell layer (GCL). Expression was higher in the central retinal region than in the retinal periphery. Using an anti-oligomer antibody we detected Aβ oligomer expression in the young, adult and old retina. Immunohistochemical labeling showed small discrete labeling of oligomers in the GCL that did not resemble plaques. Congo red staining did not result in green birefringence in any of the animals analyzed except for one old (84 months) animal. We also investigated expression of tau and phosphorylated tau. Expression was seen at all ages studied and in adults it was more consistently observed in the NFL-GCL. Hyperphosphorylated tau detected with AT8 antibody was significantly higher in the adult retina and it was localized to the GCL. We confirm for the first time that Aβ peptides and phosphorylated tau are expressed in the retina of degus. This is consistent with the proposal that AD biomarkers are present in the eye.
Conflict of interest statement
Figures

References
-
- Danesh-Meyer HV, Birch H, Ku JY, Carroll S, Gamble G (2006) Reduction of optic nerve fibers in patients with Alzheimer disease identified by laser imaging. Neurology 67: 1852–1854. - PubMed
-
- Paquet C, Boissonnot M, Roger F, Dighiero P, Gil R, Hugon J (2007) Abnormal retinal thickness in patients with mild cognitive impairment and Alzheimer's disease. Neurosci Lett 420: 97–99. - PubMed
-
- Koronyo-Hamaoui M, Koronyo Y, Ljubimov AV, Miller CA, Ko MK, Black KL, et al. (2011) Identification of amyloid plaques in retinas from Alzheimer's patients and noninvasive in vivo optical imaging of retinal plaques in a mouse model. Neuroimage 54 Suppl 1: S204–217. 10.1016/j.neuroimage.2010.06.020 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

