Tsetse Control and Gambian Sleeping Sickness; Implications for Control Strategy
- PMID: 26267814
- PMCID: PMC4580652
- DOI: 10.1371/journal.pntd.0003822
Tsetse Control and Gambian Sleeping Sickness; Implications for Control Strategy
Abstract
Background: Gambian sleeping sickness (human African trypanosomiasis, HAT) outbreaks are brought under control by case detection and treatment although it is recognised that this typically only reaches about 75% of the population. Vector control is capable of completely interrupting HAT transmission but is not used because it is considered too expensive and difficult to organise in resource-poor settings. We conducted a full scale field trial of a refined vector control technology to determine its utility in control of Gambian HAT.
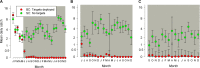
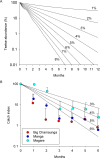
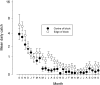
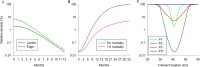
Methods and findings: The major vector of Gambian HAT is the tsetse fly Glossina fuscipes which lives in the humid zone immediately adjacent to water bodies. From a series of preliminary trials we determined the number of tiny targets required to reduce G. fuscipes populations by more than 90%. Using these data for model calibration we predicted we needed a target density of 20 per linear km of river in riverine savannah to achieve >90% tsetse control. We then carried out a full scale, 500 km2 field trial covering two HAT foci in Northern Uganda to determine the efficacy of tiny targets (overall target density 5.7/km2). In 12 months, tsetse populations declined by more than 90%. As a guide we used a published HAT transmission model and calculated that a 72% reduction in tsetse population is required to stop transmission in those settings.
Interpretation: The Ugandan census suggests population density in the HAT foci is approximately 500 per km2. The estimated cost for a single round of active case detection (excluding treatment), covering 80% of the population, is US$433,333 (WHO figures). One year of vector control organised within the country, which can completely stop HAT transmission, would cost US$42,700. The case for adding this method of vector control to case detection and treatment is strong. We outline how such a component could be organised.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures






References
-
- W.H.O. (1998) Control and surveillance of African trypanosomiasis. Report of a WHO Expert Committee. Technical Report Series 881. Geneva: World Health Organization. - PubMed
-
- W.H.O. (2013) Report of a WHO meeting on elimination of African trypanosomiasis (Trypanosoma brucei gambiense). WHO/HTM/NTD/IDM/2013.4:.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

