The octopus genome and the evolution of cephalopod neural and morphological novelties
- PMID: 26268193
- PMCID: PMC4795812
- DOI: 10.1038/nature14668
The octopus genome and the evolution of cephalopod neural and morphological novelties
Abstract
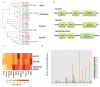
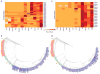
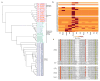
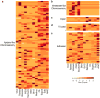
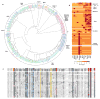
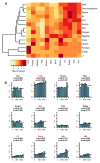
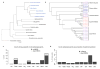
Coleoid cephalopods (octopus, squid and cuttlefish) are active, resourceful predators with a rich behavioural repertoire. They have the largest nervous systems among the invertebrates and present other striking morphological innovations including camera-like eyes, prehensile arms, a highly derived early embryogenesis and a remarkably sophisticated adaptive colouration system. To investigate the molecular bases of cephalopod brain and body innovations, we sequenced the genome and multiple transcriptomes of the California two-spot octopus, Octopus bimaculoides. We found no evidence for hypothesized whole-genome duplications in the octopus lineage. The core developmental and neuronal gene repertoire of the octopus is broadly similar to that found across invertebrate bilaterians, except for massive expansions in two gene families previously thought to be uniquely enlarged in vertebrates: the protocadherins, which regulate neuronal development, and the C2H2 superfamily of zinc-finger transcription factors. Extensive messenger RNA editing generates transcript and protein diversity in genes involved in neural excitability, as previously described, as well as in genes participating in a broad range of other cellular functions. We identified hundreds of cephalopod-specific genes, many of which showed elevated expression levels in such specialized structures as the skin, the suckers and the nervous system. Finally, we found evidence for large-scale genomic rearrangements that are closely associated with transposable element expansions. Our analysis suggests that substantial expansion of a handful of gene families, along with extensive remodelling of genome linkage and repetitive content, played a critical role in the evolution of cephalopod morphological innovations, including their large and complex nervous systems.
Conflict of interest statement
The authors declare no competing financial interests
Figures



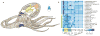

References
-
- Hanlon RT, Messenger JB. Cephalopod behaviour. Cambridge University Press; 1996.
-
- Young JZ. The anatomy of the nervous system of Octopus vulgaris. Clarendon Press; 1971.
-
- Wells MJ. Octopus: physiology and behaviour of an advanced invertebrate. Chapman and Hall; 1978.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

